11 Note: Significant diseases are indicated in bold and syndromes in italics. 1. Bacterial meningitis a. General symptoms: Only subtle behavioral changes may be apparent early in the disease course, but progression of the disease typically involves i. fever, headache, meningismus, photophobia, somnolence ii. focal neurological dysfunction (15%), particularly hearing loss iii. seizures (20%) b. general diagnostic testing i. blood cultures identify causative pathogens in 50% of cases ii. cerebrospinal fluid analysis (1) increased intracranial pressure, protein, and lactate (2) decreased glucose (3) pleocytosis is typically > 100 cells/μL, and > 60% neutrophils; may be lower early in disease course (4) Gram stain is positive in 60% (Box 11.1) (5) cultures identify causative pathogen in 75% iii. neuroimaging: usually is normal, but it may demonstrate hydrocephalus, parenchymal edema, and/or meningeal enhancement; in complicated cases, venous thrombosis or collections of pus between the dura and arachnoid layers of the meninges {empyema} may develop Yield of CSF Gram stain in bacterial meningitis is reduced only with > 2 hour of antibiotic administration. c. pathophysiology (Table 11–1) d. general treatment i. antibiotics, as in Table 11–1; adjust as the causative bacteria are identified (1) always use bactericidal agents, not bacteriostatic agents (2) meningeal inflammation makes the brain permeable to essentially all antibiotics ii. dexamethasone: reduces morbidity and mortality by 40% when used in children or adults with meningitis; give first dose prior to initiation of antibiotic therapy iii. surgical drainage of any empyema, otitis media, or sinusitis iv. prophylaxis with the Haemophilus influenza vaccine routinely in children, and with the Neisseria meningitidis vaccine in persons at high-risk for exposure e. prognosis: 20% mortality; 40% of survivors will develop seizures 2. Brain abscess a. pathophysiology: causes include i. contiguous spread: the most common route for brain abscess formation; always results in a single abscess (Box 11.2)
Infections of the Nervous System
I. Bacteria
Box 11.1
Patient group | Causative bacteria (in order) | Empiric treatment |
Neonates | Group B Streptococcus Escherichia coli Listeria monocytogenes | Ampicillin third-generation cephalosporin |
Children, adolescents, young adults | Neissera meningitidis Streptoccus pneumoniae (H. in.uenza is rare due to vaccination) | Third-generation cephalosporin ± vancomycin/rifampin |
Older adults (> 50 years of age) | Neissera meningitidis Listeria monocytogenes | Ampicillin + third-generation cephalosporin ± vancomycin/rifampin |
Immunosuppressed | Listeria monocytogenes Gram-negative bacilli Klebsiella | Ampicillin + third-generation cephalosporin ± vancomycin/rifampin |
Shunt infection | Staphylococcus epidermidis Staphylococcus aureus (methacillin resistant) | Vancomycin + third-generation cephalosporin |
Head trauma | Staphylococcus epidermidis Staphylococcus aureus Gram-negative bacilli anaerobic bacteria | Vancomycin + third-generation cephalosporin ± metronidazole |
Note: Local flora and sensitivity patterns require consultation with an infectious diseases expert.
(1) the initial infection is located in a parameningeal site, such as
(a) purulent sinusitis, which produces frontal lobe abscesses
(b) otitis media or mastoiditis, which produce temporal lobe or cerebellar abscesses
ii. hematogenous spread: abscesses preferentially target areas of old brain injury (e.g., infarction), and are multiple in 15% of cases (Box 11.3)
(1) in adults, hematogenous spread of bacteria is usually from a pulmonary infection or (less commonly) endocarditis
(2) in children, hematogenous spread of bacteria is usually from some type of cyanotic congenital heart disease that provides a hypoxic environment and a right–left shunt thereby bypassing normal lung filtration
Box 11.3
Osler-Weber-Rendu syndrome/hereditary hemorrhagic telangiectasia—pulmonary arteriovenous fistulas bypass normal lung filtration; 5% of cases develop cerebral abscesses
iii. head injury: closed head trauma is a rare cause of abscesses except in cases that involve unrepaired cerebrospinal fluid leaks
iv. neurosurgery, usually in cases where an air sinus has been opened
v. idiopathic (20%)
b. common pathogens: abscesses include multiple organisms in 90%, usually Streptococcus species (50%), Bacteroides, and Enterobacteriaceae and other anaerobic bacteria
i. abscesses from trauma also include multiple Staphylococcal species
ii. abscesses in infants often include Gram-negative bacteria
c. symptoms: headache, nausea, and somnolence caused by increased intracranial pressure focal neurological injury
i. increased intracranial pressure may enlarge the head circumference in infants
ii. fever occurs in only 50% because the infection is isolated
d. diagnostic testing: ultimately requires tissue therapy
(1) early cerebritis (stage I): exhibits poorly demarcated ring enhancement that does not wash out and parenchymal edema
(2) late cerebritis (stage II): exhibits a developing necrotic center
(3) early encapsulation (stage III): exhibits a vascularized, necrotic center
(4) late encapsulation (stage IV): exhibits a thin collagen capsule that enhances but then washes out; gliosis develops around capsule
ii. diagnosis requires tissue biopsy
iii. blood cultures are rarely helpful; avoid lumbar puncture due to herniation risk
e. treatment
i. medical treatment: most effective in neuroimaging stages I–II, before the development of an abscess capsule
(1) empiric therapy: vancomycin third-generation cephalosporin metronidazole or chloramphenicol
(2) glucocorticoids prevent fibrous encapsulation but also reduce penetration of antibiotics, therefore use only in patients who are clinically deteriorating
ii. surgical drainage in cases with mass effect, elevated intracranial pressure, poor neurological condition, or proximity of the abscess to a ventricle (i.e., to prevent ventriculitis)
3. Bacterial encephalitis: an uncommon form of bacterial infection in the brain
a. Bartonella henselae/cat scratch disease
i. pathophysiology: a Gram-negative bacillus transmitted by scratches from a colonized cat and possibly by cat fleas
ii. symptoms: fever; lymphadenopathy proximal to the scratch; encephalitis with seizures develops in only 1% of infected patients
(1) may also cause myelitis and/or radiculitis in conjunction with angiomatous skin lesions that are similar to Kaposi’s sarcoma in the immunosuppressed
iii. diagnostic testing: B. henselae enzyme linked immunosorbent assay (ELISA) or polymerase chain reaction (PCR); cerebrospinal fluid is normal in 70% and otherwise shows only a mild lymphocytosis; cultures are unrevealing
iv. treatment: gentamicin or trimethoprim-sulfamethoxazole (TMP-SMX)
v. prognosis: complete recovery in the immunocompetent
4. Bacterial vasculitis (see p. 77)
II. Mycobacteria
1. Mycobacterium tuberculosis/tuberculosis
a. pathophysiology: transmitted from person to person by infected respiratory droplets; infection of the central nervous system may develop during the initial infection (usually pulmonary) or after reactivation of a dormant infection (as in the newly immunosuppressed)
i. Mycobacterium usually spread from a peripheral site of infection to the brain by a hematogenous route or rarely by rupture of a mass of infected granulation tissue {tubercles} into the cerebrospinal fluid; may form abscesses {tuberculomas} directly in brain parenchyma as well
ii. target organs
(1) pulmonary, lymphatic, genitourinary
(2) bones and joints, including the vertebral bodies and intervertebral disks causing spondylosis {Pott’s disease}
(3) meninges > brain parenchyma
c. symptoms (neurological): neurological symptom from
i. meningitis, which has a relatively high rate of cranial neuropathy (25%, particularly CN VI > III, IV, VII) and the syndrome of inappropriate antidiuretic hormone secretion (SIADH) due to its preference for the basal meninges
(1) seizures, focal deficits (15%), and hydrocephalus may also occur
(2) can infect the spinal meninges, producing radicular and local back pain and an ascending paralysis {Foix-Alajouanine syndrome} (Box 11.4)
ii. tuberculoma development (rare), which causes focal neurological injury
iii. vasculitis (see p. 77)
Box 11.4
The Foix-Alajouanine syndrome is more commonly seen with spinal arteriovenous malformation (AVM) growth.
d. diagnostic testing: the diagnosis of tuberculosis infection in the brain is supported by identifying tuberculosis elsewhere in the body
i. for meningitis: cerebrospinal fluid demonstrates increased protein, low glucose, and a lymphocytic pleocytosis; immunosuppressed patients may not exhibit the pleocytosis
(1) elevated total protein levels (> 100 mg/dL, often > 1000) may form a web-like coagulant that is very characteristic of tuberculosis meningitis {Froin’s syndrome}; removal of the coagulant leaves a supernatant with a very low protein level
(2) cerebrospinal fluid smear is positive in only 30%; acid-fast bacilli cultures are positive in 70%, but take 8 weeks to complete
(3) M. tuberculosis PCR is positive in 75%
(4) neuroimaging may demonstrate skull base meningeal enhancement
ii. for tuberculoma
(1) neuroimaging demonstrates one or more ring-enhancing lesion (s), either high or low density
(2) tissue biopsy is required if the diagnosis of systemic tuberculosis cannot be made
e. treatment
i. medical treatment: isoniazid + rifampin + pyrazinamide ± ethambutol or streptomycin, typically continued for 6–9 months; optimal drug regimen depends upon local resistance patterns
(1) may consolidate into a two-drug regimen if the patient is clinically improved after 2 months on the four-drug regimen; continue the two-drug regimen for 10 more months
(2) vitamin B6 supplementation is necessary to prevent isoniazid neuropathy
(3) glucocorticoids can be used to control tissue edema or Froin’s syndrome that can obstruct the arachnoid granulations and cause communicating hydrocephalus
ii. surgical treatment
(1) cerebrospinal fluid shunting for any signs of hydrocephalus
(2) excision for tuberculomas that are causing symptomatic mass effect despite glucocorticoid therapy
f. prognosis: uniformly fatal within 6 weeks if untreated; 20% mortality in the immunocompetent, 30% mortality in immunosuppressed
2. Mycobacterium leprae/leprosy
a. pathophysiology: M. leprae is spread between persons by infected droplets, and it grows best at temperatures a few degrees below human body temperature; preferentially infects in the epineurium and perineurium of nerves of the extremities
i. tuberculous leprosy (in patients with strong cell-mediated immunity): a few skin nodules develop that are associated regions of sensory loss; palpable nerve hypertrophy
ii. indeterminate leprosy: a single hypopigmented patch that has sensory loss
(1) progresses to either tuberculous or lepromatous forms depending upon the effectiveness of the patient’s cell-mediated immunity
iii. lepromatous leprosy (in patients with poor cell-mediated immunity): multiple skin nodules with cartilage erosion in the nose and ears {leo-nine face deformity}; distal small-fiber sensory loss in the extremities, nose, and ears with palpable nerve hypertrophy
(1) skin nodules are not initially associated with sensory loss, as they are in the tuberculous form of leprosy
(2) weakness and arthropathy develop late in the disease course
c. diagnostic testing
i. identifying bacterial particles in macrophages in skin biopsies, nasal mucosa, or nerve biopsies
ii. lepromin skin test is useful for distinguishing between lepromatous and tuberculous forms of the disease, but is not useful in diagnosis because it is commonly negative in the lepromatous form
d. treatment: dapsone + clofazimine + rifampin; treat for 1 year in patients with indeterminate or tuberculous forms, or for 2 years in patients with lepromatous form
i. treatment often induces an erythema nodosum-like reaction upon initiation
III. Spirochetes
1. Lyme disease
a. pathophysiology: caused by Borrelia burgdorferi in North American; ticks of Ixodes (Box 11.5) genus serve as the vectors and deer serve as the reservoir
i. the host reaction is largely directed against Borrelia outer-surface proteins (Osp), which are membrane lipoproteins encoded by 21 plasmids that have high antigenic variability, which may account for the organism’s prolonged infectious course
ii. Borrelia preferentially invades meninges, nerve roots, and peripheral nerves, but only rarely brain parenchyma
b. epidemiology: common in New England, Minnesota–Wisconsin, and the Pacific Northwest
c. symptoms: 50% of infections are asymptomatic
i. stage 1 (acute infection): localized erythema migrans
ii. stage 2 (within weeks): nonspecific flu-like symptoms or meningitis; heart block and myocarditis; arthritis
iii. stage 3 (within months): mild sensory polyneuropathy; subtle cognitive disturbances occur in 5% of cases {Lyme encephalitis}
(1) radiculoneuritis is prominent only with B. afzelii or B. garinii infection, which cause Lyme disease in Europe
iv. “chronic” Lyme disease: similar to chronic fatigue syndrome and fibromyalgia, however, there is no clear evidence for its existence as a distinct disease entity
d. diagnostic testing: cerebrospinal fluid B. burgdorferi ELISA and Western blotting, or else positive blood serology in the presence of a consistent clinical syndrome
i. serum or cerebrospinal fluid Borrelia PCR is not sufficiently reliable
i. prior to development of neurological symptoms: doxycycline for 14–21 days
ii. with neurological symptoms: third-generation cephalosporin IV for 2–4 weeks
iii. prophylaxis with the Lyme disease vaccine, an OspA adjuvant
(1) single-dose doxycycline may prevent infection it administered within 72 hours of a tick bite
2. Syphilis: Caused by Treponema pallidum
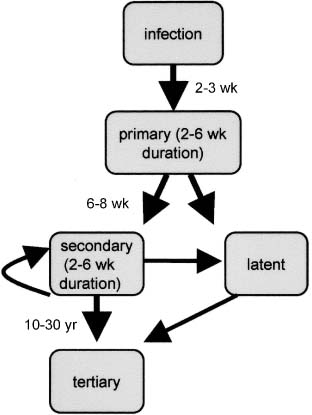
a. primary syphilis: the only symptom is a painless chancre that is acquired by contact with an actively infected site on another person (i.e., another chancre, mucous membrane or skin rash, condyloma lata)
i. may exhibit asymptomatic central nervous system infection as documented by pleocytosis of the presence of spirochetes on dark field microscopy
Figure 11–1 Stages of syphilis.
b. secondary syphilis (Fig. 11–1)
i. symptoms
(1) flu-like syndrome
(2) rash with hyperkeratosis involving the palms and soles
(3) condyloma lata, particularly during relapses
(4) meningitis in only 2% of cases, although 30% of secondary syphilis patients have an asymptomatic central nervous system infection
ii. diagnostic testing: cerebrospinal fluid evaluation by a Venereal Disease Research Laboratory (VDRL) test is specific but not sensitive, and cerebrospinal fluid fluorescent treponemal antibody (FTA) testing is sensitive but not specific; therefore, evaluate with a VDRL, but treat even if these tests are negative so long as there is pleocytosis or the presence of oligoclonal bands in a patient with a compatible clinical syndrome
(1) baseline cerebrospinal fluid cell count, protein level, and VDRL titer should be obtained to compare with follow-up tests to assess the adequacy of treatment
iii. treatment: penicillin G 4 × 106 U IV q.4.h. for 14 days
(1) to determine the adequacy of treatment, reexamine cerebrospinal fluid every 6 months for 3 years whereas successful treatment is defined as
(a) reduction in the lymphocyte pleocytosis and protein level, which are the most sensitive and rapidly changing indicators of treatment efficacy
(b) fourfold reduction in VDRL titer, which may take 1 year to achieve
(2) repeated treatment is indicated if the cerebrospinal fluid studies fail to normalize or if symptoms persist
(3) in penicillin-allergic patients, attempt penicillin desensitization or else use doxycycline for 4 weeks
c. latent syphilis: an asymptomatic central nervous system infection that can be demonstrated in 40% of cases
i. diagnostic testing: as above; obtain baseline cerebrospinal fluid studies to assess adequacy of treatment, as per secondary syphilis
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree