TMCWG
Complete acute transverse myelitis
Acute partial transverse myelitis
Deficits attributable to the spinal cord
Sensory, motor, or autonomic dysfunction
Moderate or severe symmetrical weakness and autonomic (bladder) dysfunction
Mild sensory and motor dysfunction, bilateral or unilateral; when severe, marked asymmetry is observed
Bilateral signs and/or symptoms (not necessarily symmetric), clearly defined sensory level
Symmetric sensory level
Sensory signs or symptoms attributable to a sensory level or hemi-level or MR lesion typical of myelitis
Signs of inflammation
Demonstrated by CSF findings or Gd.-enhanced MRI
CSF or MRI evidence of inflammation within the spinal cord may or may not be present
Development
Progression to nadir between 4 h and 21 days following the onset of symptoms
Exclusion criteria
Radiation within 10 years, vascular disease, brain abnormalities suggestive of MS or other demyelinating disease, presence of NMO, ADEM, connective tissue diseases, sarcoidosis and proven infection with known agents
The tasks of the radiological department at this point are first to rule out cord compression or tumor. Second, infarction and vascular malformation are to be differentiated. Third, the advantage of MRI as a tool to detect and differentiate inflammatory lesions in the spinal cord and elsewhere has become apparent and appreciated. This chapter aims to describe those findings and the underlying diseases. Because the diseases represent great variety, the literature cited may seem extensive – nevertheless a simplified classification of spinal cord inflammation has been used.
15.1.1 Preliminary Remarks
Scott [1] proposes further differentiation of the spinal cord syndrome (Table 15.1). The mere description gives some clues as to the further course:
The rate of transition to clinically definite multiple sclerosis (CDMS) in patients presenting with a complete transverse myelitis as defined in Table 15.1 seems to be less than 2 %. In cases following the definition of an acute partial transverse myelitis, the rate of conversion to CDMS is estimated to be anywhere between 20 and 30 % at approximately 5 years follow-up – despite negative cerebral MRI [1]. Likewise, severe initial symptoms suggesting spinal shock seem to be highly predictive of a poor outcome [4].
From a radiological point of view, it is worth noting that clinical diagnosis of myelopathy is not always straightforward:
1.
There are symptoms which are ambiguous by nature. Weakness of the lower extremities may be caused by disease of the muscle, the nerves or the neuromuscular junction. Muscle stiffness resulting from an autoimmune or paraneoplastic syndrome could be mistaken for spasticity, and gait disturbances of bilateral frontal lobe lesions may mimic myelopathy.
2.
One should take into account that there may be some overlap between syndromes. For example, two prototypical acute inflammatory diseases, transverse myelitis, and polyradiculitis (Guillain–Barré–Strohl syndrome) have heralding infection, ascending symptoms, pain and an elevated protein content of cerebrospinal fluid in common. Moreover, simultaneous inflammation of spinal cord and roots seems to occur in several cases [5].
3.
Chronic but hitherto undetected disease can show sudden worsening, often precipitated by viral illness or minor trauma. Examples of this include primary progressive multiple sclerosis, motor neuron disease including primary lateral sclerosis, vitamin B12 or copper deficiency myelopathy ([3]; see Schmalstieg in Chap. 14). Therefore it is not infrequent that imaging leads to surprising findings which stimulate the clinician to targeted history taking.
4.
The above-mentioned definition of the TMCWG rules out cases which reach the maximum deficit within less than 4 h. As a result, only a few cases of peracute idiopathic myelitis may be excluded, but it is likely that some vascular myelopathies fulfill the given time criterion for myelitis. Spinal infarction may even show some contrast enhancement if scanned repeatedly as proposed to demonstrate inflammation. Nevertheless, signs which strongly indicate myelopathy form the criteria embraced in Table 15.1.
ATM has an incidence rate of one to four new cases per million people per year (TMCWG), roughly a fifth occurring in the young, below the age of 18 years [6]. One small, more recent study from North Canterbury, New Zealand – a region with a known high incidence of multiple sclerosis (MS) – differentiates between acute complete and partial myelitis, giving an annual incidence of 6.2 cases per million for the first and of 24.6 cases per million for both complete and partial myelitis [7].
Whenever acute and subacute myelopathies are taken as a whole, non- inflammatory etiologies (i.e. vascular, postradiation and toxic) are to be taken into consideration as well. As shown by a large French series of 170 consecutive cases, a definite diagnosis is determined in part by observation of the further course of the disease: 55 of 101 (54.4 %) myelopathies initially classified as uncertain of cause were ultimately diagnosed as multiple sclerosis (n = 45), systemic disease (n = 5), neuromyelitis optica (n = 5) [2].
Spinal cord lesions as depicted by MRI are highly unusual in healthy individuals including older age groups (Thorpe 1993, in [20]) and thus the demonstration of intramedullary abnormality is of high value in a newly evolving disease.
On the other hand, one has to take into account that the detection rate for spinal lesions is far from ideal, because of motion artifacts and resolution with an insufficient discrimination of detail on axial slices [8]. A comparison of lesion numbers and volumes on images from 1.5 and 3 T scans of MS has concluded that there seems to be no real gain from higher field strength [9].
High sensitivity of spinal MRI to detect ATM
In the case of ATM, however, sensitivity seems to be high: only 2 out of 38 cases in a series of children and adolescents recruited between 2000 and 2004 showed a normal MRI scan of the spinal cord [6]. A large multicenter study of ATM in adults, where patients were recruited between 1994 and 2004, gives a detection rate of 43/45 (95.2 %) for ATM lesions [4].
The possibility remains that there are more complex acute cases ([5], see Sect. 15.3.4), and in subacute or chronic myelopathy there is additional ambiguity in attempting to recognize a clear-cut abnormality (see [20]). Moreover, the abundance of vertebral changes and the ease with which osteophytic compression of the spinal cord is depicted by MRI altogether encompass the risk of mistaking those findings as the most relevant, disregarding more subtle or questionable intramedullary alterations.
15.2 Myelopathies Associated with Infection
Myelopathies associated with infection are rare when considering the rough incidence numbers (given above) for both complete and partial myelitis. Debette et al. [2], investigating the course of acute and subacute myelopathies, consolidated all infection-associated cases into the one heading of “parainfectious myelopathy” and estimated that 12.4 % (21/170) belong in this group. It is pertinent to note that in all of these rare instances a timely and accurate diagnosis is crucial to improve outcome.
Infections affecting the spinal cord lead to signs and symptoms in different ways: their consequences may be caused by cytolysis of neural cells induced by viral invasion, by destructive bacterial inflammation, by space-occupying effects as in the case of granuloma or by vascular complications. However, even in the case of proof for an infectious agent in CSF, the interpretation of the phenomena should extend beyond that of mere local tissue destruction, and instead take into account molecular mimicry, superantigen-mediated inflammation or humoral derangements as well [10]. For instance, the development of paraplegia within hours and the diffuse extension of signal abnormality over large parts or the entire length of the spinal cord could advocate in favor of an immunopathogenesis. The term ‘myelopathy’ is chosen as an umbrella term, encompassing direct tissue damage on the one hand, and more complex, mostly concealed steps in immunopathogenesis on the other. Table 15.2 provides a short and inevitably incomplete overview of infectious agents which are named with respect to frequency, predilection, or therapeutic significance. Myelitis is viewed as an emergency case in the clinic and for the calculation of antimicrobial therapy some very unusual agents must also be considered, depending on immuno competence and epidemiological factors. For instance, Listeria monocytogenes is a widely unknown cause of myelitis in most parts of the world, but, if present, some standard antibiotics fail as it is an intracellularly replicating bacterium.
Table 15.2
Infectious myelopathies –agents
Viruses: | DNA: | Herpesviruses (HSV-2, HSV-1, VZV, CMV, Epstein-Barr, HHV-6, -7) |
RNA: | Flaviviruses (Japanese e., St. Louis e., Tick-borne e., Dengue v., West-Nile v.) | |
Orthomyxoviruses (Influenza A) | ||
Paramyxoviruses (Measles, Mumps) | ||
Picornaviruses (Coxsackievv., Echovv., Enterovv. Hepatitis A,C, Poliovv., vaccine- associated: Sabin) | ||
Retroviruses: | HIV, HTLV-1 (chronic myelopathies) | |
Bacterial: | Mykoplasma pneumonia | |
Borrelia b., Treponema p., Mycobact. tuberc., Tropheryma whipple | ||
Listeria, Brucella (contaminated dairy products) | ||
Staphylococcus, Streptococcus., Escherichia c., (also:) | ||
Nocardia, Actinomyces | ||
Fungal: | Blastomyces, Coccidioides, Aspergillus, Candida | |
Parasites: | Bilharziosis (Schistosomiasis) (Far East, South America, Africa), | |
Neurocysticercosis (China, Southeast Asia, India, Mexico, Central, South America) | ||
Gnathostomiasis (Southeast Asia) | ||
Strongyloides (tropical, subtropical areas, southern United States) | ||
Toxocara (worldwide, housing of dog) | ||
Toxoplasmosis (AIDS) | ||
15.2.1 Known Infectious Agents Associated with Acute Myelopathy, Viruses
Viruses are the most common agent causing acute infectious myelopathy [2], but definite proof by serological tests and polymerase chain reactions more often fails to give a definite diagnosis [3, 11]. Given the definition above, all such cases could be viewed as “idiopathic” ATM [3]; see Sect. 15.2.4. On the other hand, because of the implementation of novel diagnostic techniques in the last two decades, the spectrum of diseases caused by infection from known viral agents have been able to be better characterized [12].
The most common neurologic disease caused by any of the herpes viruses is zoster. Infection with the varicella zoster virus (VZV) usually takes place during the first two to three decades of life, leading to varicella (chicken pox). The viral genome persists in the dorsal root ganglia (the trigeminal or geniculate ganglia as well) and may be reactivated later in life, then causing shingles. Typically, individuals above 60 years of age and immunocompromised patients become ill. Both chicken pox and shingles may be complicated by neurological diseases including myelitis [13]. In the latter case, a partial transversal syndrome may result days to weeks after a painful rash in one or several dermatomas, caused by spreading of the virus from the spinal ganglion. If sensation is impaired bilaterally, the upper level should be compatible with the segment of VZV reactivation. Dermatomal pain syndromes caused by VZV from reactivation in thoracal dorsal root ganglia, but without rash (zoster sine herpete) might be under-recognized.
Imaging. In the acute stage, more extensive alterations may dominate longitudinally [3] and intramedullary, as well as the occurrence of radicular enhancement [13]. If remote spinal lesions ensue, they are interpreted as a consequence of multifocal small vessel vasculitis, thought to be caused by the hematogenous spread.
At times, especially in the further course of the disease, lesions can be recognized as being more circumscribed, referring to implicated roots (Fig. 15.1, Table 15.3). Recurrent protean manifestations of VZV infection (meningoencephalitis, vasculopathy, myelitis) may occur with or without a rash.
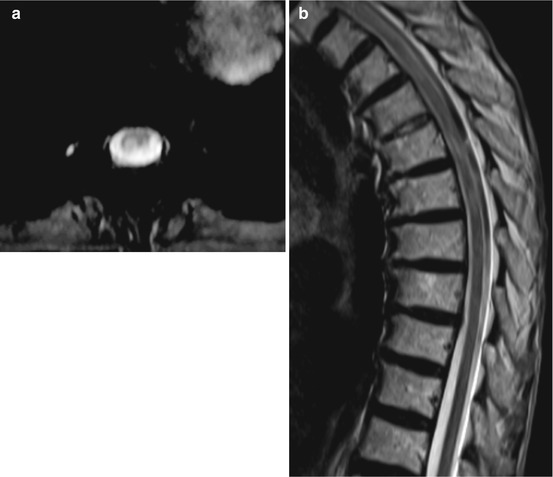

Fig. 15.1
A 77-year-old female with chronic state after zoster in the dermatomes D 6 and D 7 and with gait disturbance. Sagittal T2WI (a) and axial T2*WI (b) showing signal increase of the dorsal spinal cord. At the time of exanthema there was no gait disturbance
Table 15.3
ATM – five imaging findings that lessen differential diagnoses
Segmental distribution (Fig. 15.1): |
Intramedullar lesions affecting white and gray matter, corresponding to dermatomal rash or pain |
Varicella- Zoster virus (VZV) |
DD: HSV- 2, without rash: diverse! |
“Poliolike”: (Fig. 15.3) |
Lesions affecting the gray matter of the anterior horn and surroundings |
Polio-, St. Louis-, West-Nile-, FSME- viruses |
DD: spinal infarction, “Kira Hopkins Syndrome” |
Enhancing nodular lesions, located in the leptomeninx and/or intramedullary, possibly T2 iso- or hypointensity |
DD: sarcoidosis, tuberculosis, lues cerebrospinalis, parasitic infections, metastasis |
Lesions with extensive homogenous T2-hyperintensity lengthwise, affecting the central gray matter structures, often with some swelling. In case of NMO enhancement is more frequently intense and homogeneous |
DD: viral myelitides, NMO, SAD, ADEM, Gnathostomiasis, spinal vascular disease (see Chap. 17) |
Herpes simplex virus (HSV) -2, the etiological agent of genital herpes, is known to be a common cause of aseptic meningitis in adult women, often occurring without a rash. It also accounts for 6 % of necrotizing frontotemporal encephalitis and has been reported to lead to sacral radiculitis [11] as well as to acute and subacute myelitis (Nakajima 1998, cited in [12, 13]). Typically, a corresponding localized dermatomal rash occurs [13]. First symptoms of this necrotizing myelitis may be sensorimotor disturbances of the lower extremities and bladder dysfunction, followed by ascension over the course of several weeks. Prognosis varies, in some cases leading to tetraplegia and death despite timely therapy. Moreover, recurrences have been reported.
HSV-1 causes the classic necrotizing encephalitis (which spreads from the temporal lobe) and, less frequently, brainstem encephalitis. Besides this, HSV-1 may lead to an acute or subacute myelitis in immunocompetent adults, but, as reported, less frequently than HSV-2. Nakajima et al. cited in [12, 13]) demonstrated a parenchymal high signal both on T1WI and T2WI, suggesting hemorrhagic necrosis as known from temporal HSV-1 encephalitis.
Imaging. MR imaging may demonstrate longitudinally extensive T2-hyperintense lesions (longitudinally extensive transverse myelitis, (LETM), Table 15.3) with variable intra-medullary and, possibly, meningeal and radicular enhancement on contrast-enhanced T1WI [14].
In HIV patients as well as in healthy adults, the cytomegalovirus (CMV) is certainly highly prevalent, but remains asymptomatic. In the case of infection or reactivation, it causes retinitis or encephalitis selectively in the immunocompromised, evidenced by enhancement along the ventricular border, polyradiculitis, multifocal neuropathy and myeloradiculitis, often concomitantly [12, 13]. Among spinal diseases which are known to complicate AIDS, CMV-radiculitis was the most common intradural disease (Thurnher, cited in [14]).
Imaging. Typically thickening, clumping and enhancement of nerve roots ensue, quite similar to what is shown in Guillain–Barre syndrome (see Fig. 13.7), often with involvement of the conus as well [13, 14].
CMV myelitis may occur in isolation: a case of Moulignier et al. (cited by [13]) showed some swelling in the conus medullaris and ring enhancement intramedullary, mimicking a tumor. CSF analysis exhibited pleocytosis. Moreover, Moulignier et al. mention a 7-year-old, perinatally HIV infected girl with a similar lesion in the cervical spinal cord. Histology provided evidence of necrotizing myelitis and positive CMV immunostaining, but the spinal roots did not show any signs of inflammation.
In contrast, immunocompetent adults manifesting neurological disease associated with CMV are rare, occurring as transverse myelitis or myeloradiculitis. Concerning the pathogenesis, some scattered case reports give the impression of considerable heterogeneity, with negative PCR when tested in the CSF, and several normal MR reports. Therefore Fux et al. [15] argued in favor of an immune-mediated rather than a directly virus-induced pathogenesis and pointed to the absence of intrathecal production of specific CMV antibodies in their particular case. Karacostas et al. [16] reported on a 16-year-old student who developed paraplegia days after a temporary improvement of an illness starting with a sore throat, malaise and fever. T2WI demonstrated an increased signal from the lower cervical to the thoracic cord (LETM). Contrast-enhanced MRI was unrevealing and the further course was very favorable, showing complete recovery within 2 months. In the CSF, the cell count was normal and no viral DNA was detected. Again, this case could serve as an example for a probable immune-mediated pathogenesis (see Sect. 15.2.5).
The Epstein–Barr virus (EBV) causes infectious mononucleosis, which is complicated by neurological manifestations in 1–5 % of all cases, whereby this rate may possibly be an underestimation [13]. Severe complications are rare, but protean: among others, they include meningoencephalitis, acute disseminated encephalomyelitis (ADEM), radiculitis, encephalo-myeloradiculitis and myelitis [12, 13].
Imaging. Potential patterns of radiculitis on contrast-enhanced T1WI include leptomeningeal and root enhancement along the intrathecal course of spinal nerves, with or without thickening.
These findings are preferentially depicted with fat suppressed T1WI after intravenous application of gadolinium (see Chap. 13).
The pathogenesis of inflammatory CNS-disease associated with EBV infection is controversial, either favoring a direct virus invasion, or an immune-mediated lesion caused by cytotoxic CD 8+ cells [13]. An example possibly supporting the first interpretation is given by Gruhn [17]: a 16-year-old boy had received unrelated bone marrow transplantation. Nineteen months later he presented with a partial transversal syndrome and pleocytosis; PCR for EBV was positive in the CSF. On T2WI an intramedullary edema throughout the cervical and thoracic cord was demonstrated with some spotty hyperintensities which showed strong enhancement on contrast-enhanced T1WI. They were interpreted as inflammatory foci by the authors. The boy was treated with gancyclovir and hyperimmune globulin and recovered completely. An example of postinfectious EBV myelitis has been presented by de Seze et al. [4], corresponding to a long-segment diffuse T2 hyperintensity cervicodorsal (LETM, Table 15.3). The case imaged with Fig. 15.2 was interpreted as infection with HHV-6, proven by PCR, again showing LETM.
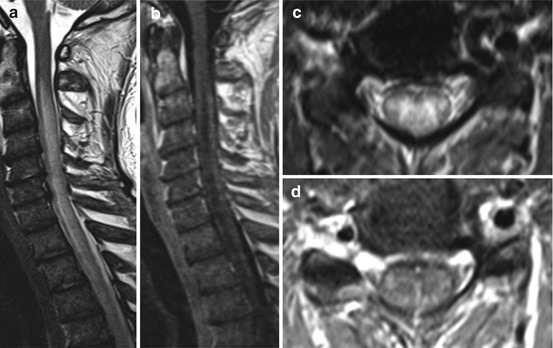

Fig. 15.2
HHV 6 infection. A 47-year-old man with acute lymphocytic leukemia, bone marrow transplantation 5 months ago and ataxia. Sagittal T2 WI (a) reveal extended hyperintense signal changes especially in the cervical spinal cord pronounced in the dorsal columns (c; axial T2 WI) with mild swelling (a, c) and slight enhancement (b, d: sagittal and axial T1 WI post contrast (pc) medium administration)
If in the context of viral infection rapidly flaccid paresis arise in the absence of sensory disturbances, either radiculitis or a “poliomyelitis-like” syndrome might be the cause. In such cases proof of a spinal lesion is of interest.
Poliomyelitis is nearly eradicated and today only occasional cases are reported in association with oral vaccination.
Imaging. MRI shows obvious similarities to ischemic or degenerative loss of anterior horn cells (see Figs. 20.7 and 20.8). Lesions are radiologically and neuropathologically confined to the anterior two-thirds of the spinal gray matter [18]. Similarities to these patterns are seen in infections by some flaviviruses and enteroviruses [3].
In such cases the clinical syndrome and imaging studies suggest a preferential rather than an isolated destruction of anterior horn cells (Fig. 15.3, Table 15.3).
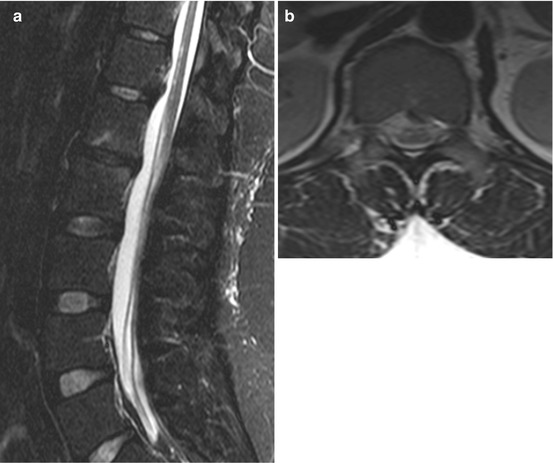

Fig. 15.3
Chronic state after tick-borne myelitis at the age of 13. Sensory level at dermatome 9, initially flaccid paralysis and absent tendon reflexes of the legs. Later on, walking with minimal restrictions, but plantar response being extensor. Sagittal (a) and axial T2 WI (b) showing ventral accentuated slight hyperintense lesions
Equally, some similarities are given to Hopkins syndrome and atopic myelitis. The former was described as a “poliomyelitis-like” condition following asthma attacks, associated with elevated serum IgG levels. Affected children (and in a few cases adults) showed an acute onset of flaccid pareses of one or two limbs, days to weeks after an asthma attack [19].
In recent years the flavivirus West Nile virus, predominantly transmitted by mosquitoes, has attracted attention, causing outbreaks with higher incidence of severe neuroinvasive disease in North America, totaling 8,606 cases between 1999 and 2005. As further detailed in a review of Kramer [20], the virus was first isolated in 1937 in the West Nile province of Uganda and has spread rapidly throughout the Western world. While the majority of infected individuals stay asymptomatic, less than 1 % develops meningitis, encephalitis or flaccid paralysis, sometimes overlapping in the same patient. However, flaccid paralysis may occur in the absence of meningitis or an encephalitis syndrome as well. Pareses are often asymmetric, reaching a nadir within hours. Bladder and bowel function are disturbed in some patients, but on neurological examination substantial sensory deficits might be absent.
Imaging: Focal changes have been described on cerebral MRI scans: these are patchy and located in the white matter, but can affect pons, substantia nigra and the thalami as well, and may result from cytolytic effects of the virus. Spinal MRI may show gray matter lesions of the anterior horn, documented by prolonged T2-relaxation time, uni- or bilaterally, possibly longitudinally extended. Occasionally, spinal root or patchy parenchymal enhancement is reported [14].
Of course, referring to the above-mentioned question, only the gray matter findings are decisive. Interestingly, as shown with another flavivirus infection, the appearance of brain abnormalities later in the course of spinal disease may indicate ADEM as well [21].
15.2.2 Known Infectious Agents Associated with Subacute Myelopathy, Bacteria
Bacterial inflammation of the spinal cord parenchyma may spread from bacterial meningitis as described in Chap. 13, hematogenous or from adjacent infection, forming an abscess, granulomas, or causing vascular damage (see Chap. 13). However, such cases represent rarities, as stated by Kurita et al. [22], who collected 25 well-documented cases of intramedullary spinal cord abscesses worldwide within 10 years. The most common presentation was deficit occurring in all patients, followed by fever, pain, and bladder disturbances. The radiological findings were similar to those known from brain abscesses.
Moreover, as discussed in Sect. 15.2.5, bacteria may act abruptly and “parainfectiously” on the spinal cord, a process which is poorly understood.
More often observed are spinal cord syndromes caused by bacterial inflammation, taking a more protracted course, as in borreliosis or tuberculosis.
Lyme disease, or borreliosis, are the most frequently recognized arthropod-borne infections of the nervous system in the USA and in Europe. Upwards of 25 % of the healthy European population is seropositive for anti-borreliosis antibodies, yet has never been sick with the disease. Proof of an infection affecting the central nervous system therefore rests on examination of the cerebrospinal fluid. In Europe, meningopolyradiculitis represents the manifestation most often seen.
Imaging. On T1WI a prominent enhancement in the leptomeninges is to be expected, and radicular enhancement is even more typical for borreliosis.
Several case reports of “myelitis” highlight aspects of pathogenesis and are of interest for differential diagnosis: described and displayed are (1) a prominent long segment swelling of the spinal cord in a 12year-old child, even without neurological deficit, possibly with some patchy intramedullary enhancement [23], (2) a circumscribed, prominent intramedullary enhancement surrounded by swelling [24], and (3) a painless “polyomyelitis-like syndrome” of the upper extremities [25], together with the well-known meningeal and radicular enhancements. In this particular case a T2-hyperintense, enhancing lesion was identified, exactly confined to the cervical anterior horn bilaterally, extending in longitudinal direction only.
More typically, patients complain of persistent, radiating pain as in the case displayed (Fig. 15.4). Therapy leads to cure and subtle spinal symptoms are easily missed – therefore the incidence of myelitis remains unclear.
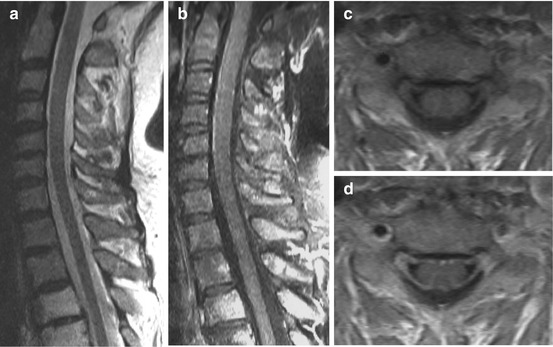

Fig. 15.4
Borreliosis in a 67-year-old female. Numbness, pain, and flaccid paraparesis in both arms and unsteady gait for 2 weeks. Sagittal T2 WI (a) showing no clear-cut intramedullary abnormalities, but T1 WI pc (b, sagittal; d, axial) disclosed thickened and enhancing roots (c: T1 WI axial)
The other well-known spirochaetal disease with prominent neurological sequelae is syphilis.
In an attempt to cover a rare illness with diverse manifestations, some simplification seems inevitable. Therefore, following Conde-Sendin [26], meningeal, meningovascular and ocular forms are considered early and general paresis (progressive paralysis) and tabes dorsalis late forms of syphilis. Accordingly, myelitis is classified as early and dealt with in this section, but tabes dorsalis is viewed as chronic and covered under Sect. 15.2.6. These authors report an incidence number for new cases of syphilis of 1.77/100,000 over the year 2000 in Spain.
Meningeal inflammation, the formation of granuloma (gumma), infarction as a consequence of arteritis and myelitis may occur concurrently. Concerning meningomyelitis, latencies varying from 1 to 30 years from the onset of infection are given [11]. The radiological spectrum of meningomyelitis may well be illustrated by scattered case reports, again with respect to differential diagnosis: in a 25-year-old patient, a small space-occupying lesion in the dorsal cervical spinal cord caused a partial transversal (Brown Sequard) syndrome and was interpreted as an isolated demyelination, where brain MRI was unremarkable. Based on a biopsy 2 years later, the lesion was diagnosed as gumma [27]. On T2WI the center of the nodule was isointense. In the case of Tsui et al. [28], brain MRI was normal as well: a 57-year-old woman developed an acute complete transversal syndrome within 5 days. An extensive high signal involving the whole spinal cord was present on T2WI. A nodular hypointensity in the thoracic cord was depicted (possibly gumma) but not commented on. This small mass was surrounded by focal enhancement, and CSF pleocytosis was shown. Kikuchi et al. [29] described a case of syphilitic meningomyelitis. A 36-year-old man first evolved a cervical pain and, within months, weakness and sensory loss in the upper extremities, followed by difficulty in walking and pyramidal signs on both sides at the time of neurological evaluation. Spinal cord MRI revealed swelling of the entire spinal cord and the leptomeninges showed enhancement. The superficial parts of C3 to 5 and Th3 to 4 showed intense enhancement, giving a “candle guttering appearance” on sagittal images. The abnormal enhancing parenchyma showed hypointensity in T2WI, named by the authors as “flip-flop sign”. The latter finding may be interpreted as caused by lipids and the release of free radicals by macrophages, corresponding to the composition of granulomata. Unfortunately, axial slices were neither described nor displayed. Brain MRI showed two small intensely enhancing lesions. All three individuals described were immunocompetent. In the case of pathological examination, luetic meningomyelitis shows thickened, inflamed meninges with predominance at the cervical region (hypertrophic pachymeningitis, [11]), a description fitting the findings mentioned above quite well. To sum up, syphilis may lead to an inflammation resembling other granulomatous diseases (see sarcoidosis, Sect. 15.3.5, Table 15.3).
Authors from Pakistan [30] who reported a prevalence of 405/100,000 for tuberculosis in their country, compared clinical and neuroimaging features of ten of their own patients with tuberculous myelitis. Most patients presented as radiculomeningitis (Figs. 13.4 and 13.5), but isolated myelitis or polyradiculitis are not uncommon.
Imaging. The involvement of spinal cord may resemble glioma or lymphoma, more than 80 % affecting the thoracic spine. Besides intramedullary localization, tuberculoma intradural-extramedullary and isolated involvement of the cauda equina were observed. The MRI signal characteristics of tubercular lesions are related to the histological stage, T1-hyperintensity and T2 shortening being representative of caseous necrosis with a large amount of lipids and macrophages. A majority of the lesions variably enhance contrast on T1WI, showing diffuse, nodular, ring-like or tumor-like patterns, but the absence of enhancement does not exclude the disease.
Spinal cord tuberculoma usually responds to medical treatment; syrinx formation, spinal infarction and atrophy do occur in the course of the disease.
15.2.3 Known Infectious Agents Associated with Subacute Myelopathy, Fungi, Parasites
Fungi as a cause of myelitis may occur during the course of known diseases. Cryptococcus neoformans, for instance, is known to be a prominent source of opportunistic infections in patients suffering from AIDS, but they play no role in presenting spinal symptoms in developed countries and thus are not addressed here (see [11, 31]).
Likewise, exposure to parasitic diseases is limited in developed regions, but other than fungi, parasites may cause serious infection in immunocompetent individuals. In times of mass tourism and globalization, they are worth mentioning with some degree of importance not only in the countries where they are endemic. Some index of suspicion is given by contexts outlined in Table 15.2. Another hint may result from eosinophilia in the blood or CSF.
In cysticercosis, spinal involvement rarely occurs, below 5 % [14] of all cases. Surprisingly in a series of 16 cases with spinal cysticercosis from four centers [32], all had evidence of simultaneous cranial manifestations.
Imaging. The patients with intramedullary involvement showed either solitary focal cystic lesions within the spinal cord (6/16), or syrinx formation related to chronic arachnoiditis (2/16). Evidence of intradural-extramedullary disease included cystic structures within the subarachnoid space or meningeal enhancement.
Saleem et al. [33] from Egypt were able to present eight cases of spinal schistosomiasis with pathological correlation. All patients were male and the site of involvement was the lumbar intumescence of the spinal cord in all cases.
Imaging. Involvement of the lumbar intumescence with swelling and intramedullary nodular enhancement, correlating to multiple schistosomiasis microtubercles. Meningeal enhancement was present in all and some radicular enhancement in four cases.
A similar striking male preponderance in another series [34] was believed to result from greater exposure of men to schistosomal infestation in agriculture. The invariable involvement of the lumbar cord is explained by the existence of valve free venous plexus anastomoses between intraabdominal and spinal veins and raised intraabdominal pressure, which permits the eggs of the worm to migrate through these vessels. The highly antigenic structure of the ova provides an extensive immune reaction.
A common cause of paraparesis with eosinophilic CSF in Northeast Thailand is the infection with Gnathostomiasis, caused by the consumption of raw fish or other intermediate hosts. Depicted in a case report of [35] is diffuse cord enlargement with high signal on T2WI of the cervical and thoracic spinal cord, encompassing the gray matter. Small intracerebral or subarachnoid hemorrhages seem to be common.
In a case of toxocara infection (Fig. 15.5) [36] there were several, areas of raised T2 signal intramedullary and leptomeningeal as well as some punctuate intramedullary enhancement. Diagnostic confirmation in such cases depends on positive CSF or serum antibody titers.
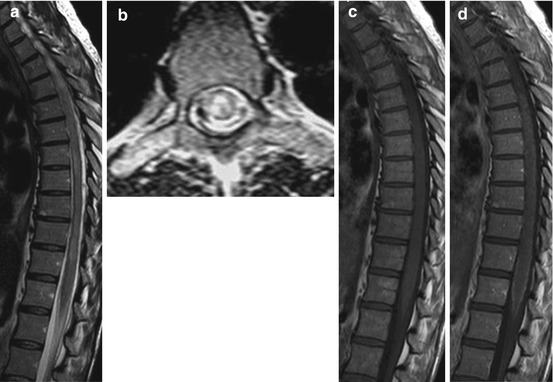

Fig. 15.5
A 44-year-old woman (dog owner) with eosinophilic meningomyelitis and progressive paraparesis caused by parasitic infection (neurotoxocariosis). Spinal MRI showing hyperintense lesions of the thoracic cord (a, b; sagittal and axial T2WI) with diffuse leptomeningeal and faint punctuate intraparenchymal contrast enhancement (c, d; sagittal T1WI)
15.2.4 Idiopathic Acute Transverse Myelitis (Possible, but not Proven Virus Infection)
Applying the definition of idiopathic ATM, some cases are to be characterized that differ neither in clinical nor in radiological detail from infections with proven viral etiology [3, 4, 14], begging the question whether the causative agent is merely missed or unknown.
In 2002 the TMCWG addressed the problem whereby neuromyelitis optica (Devic’s disease; see Sect. 15.3.3) could not be differentiated sufficiently. In the meantime, a potent serological marker was found and recent series of ATM [6] rule out neuromyelitis optica not only by follow-up of the patients but by defining aquaporin-4 antibody.
Lastly, the question as to whether there is a pure spinal form of acute disseminated encephalo-myelitis fulfilling the definition of idiopathic ATM remains unanswered and are discussed in Sect. 15.3.4. Bearing this in mind, neither heterogeneity of outcome nor a diversity of radiological features [14] in this group is surprising. The moderate detection rate of earlier series might be explicable by technical limitations of older MR scanners, so the notion of Goh et al. [14]: “MR imaging demonstrates T2-hyperintense lesions in almost all reported cases” could be the adequate description of currently available imaging results.
Imaging. T2-hyperintense lesions are seen in almost all cases with extension lengthwise over more than three segments, centrally located and involving most of the cross section (LETM). The cervical or thoracic spinal cord, and less often lumbar or several parts, are involved.
15.2.5 Parainfectious Myelitis, Post-infectious Myelitis (see Sect. 15.3.4)
The terms ‘parainfectious,’ and likewise ‘post-infectious’ myelitis, are used to stress the point that the causative agents for these spinal cord syndromes are factors other than the direct destructive outcomes of an infectious agent [10, 11]. In fact, trigger and effector mechanisms in respective cases are not well-known and cytolitic viral action vs neural injury resulting from immune-mediated mechanism may be impossible to differentiate on clinical grounds [10, 14]. Some examples given above show there may be blurring margins even in established etiologies. The terms are descriptive and used when a close temporal relationship exists between a systemic infection and the acute or subacute occurrence of myelitis [10].
An example of parainfectious myelitis – as well as the technical problems of imaging – is given in Fig. 15.6: during an acute septicemia with Staphylococcus, a transversal syndrome evolved within hours. The first MRI study was performed about 2 h after complete paraplegia, which did not show any substantial improvement during the disease progression. This rapid catastrophic course was possibly elicited by microbial superantigens [10]. Another case (Fig. 15.7) made a favorable recovery in spite of early and extended radiological findings corresponding to LETM.
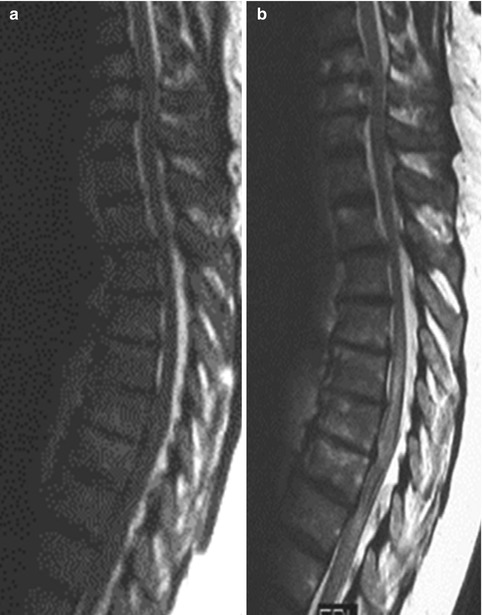
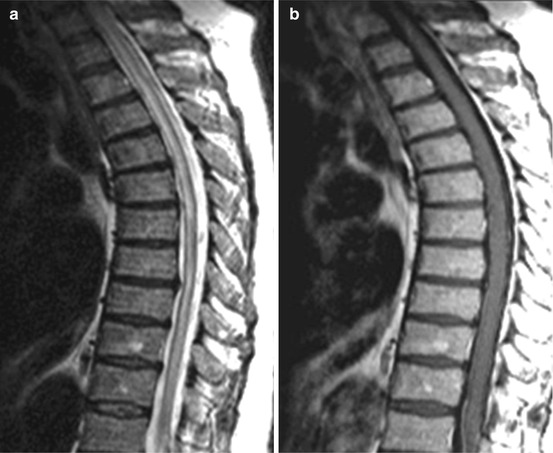

Fig. 15.6
Parainfectious myelitis in a 62-year-old woman with complete paraplegia within less than 4 h. Cerebrospinal fluid (CSF) showed pleocytosis, cultures from blood and spinal fluid positive for Staphylococcus. First MRI (a, T2 WI sag.) was performed 2 h after paraplegia and second MRI (b, T2 WI sag.) 4 days later, revealing swelling and long sectional hyperintense signal of the thoracolumbar spinal cord

Fig. 15.7
Longitudinally extended transverse myelitis (LETM) 1 day after incomplete paraparesis during urosepsis (Table 15.3), e.g. parainfectious myelitis. Sagittal T2WI (a) showing a central accentuated hyperintense lesion over the whole thoracolumbar spinal cord without intramedullary enhancement (b, T1 WI sag. pc)
15.2.6 Myelopathy Associated with Subacute and Chronic Infections
The most commonly observed spinal cord lesion of AIDS patients on necropsy is vacuolar myelopathy (VM). This is in contrast to clinical series where apparent myelopathy is an infrequent experience in the order of 1 % of neurological complications [11]. Although VM is notoriously linked with the AIDS pandemic, it has been observed in patients with cancer and other immuno-suppressive conditions [11]. Accordingly, VM occurs independently from HIV infection of the spinal cord parenchyma and highly active antiretroviral therapy does not seem to alter the course. VM shows striking similarities to the subacute degeneration of the spinal cord following vitamin B12 deficiency, and, because both conditions probably have parts of their pathogenesis in common, they are discussed together in Sect. 16.3.
HIV myelitis must be taken into consideration, when under circumstances of known or suspected HIV infection a myelopathy appears, probably with a more protracted course than with an infection caused by the various above-mentioned agents.
Imaging. In contrast to VM, HIV myelitis shows parenchymal and leptomeningeal enhancement without swelling [38, 14].
Notably, in the case report of Michou et al. [38], highly active antiretroviral therapy showed efficacy.
Another retrovirus, Human T-cell Lymphotrophic Virus (HTLV), poses a health problem affecting high-risk individuals and those in endemic areas such as southern Japan, equatorial Africa, the Caribbean, and parts of South America. It is transmitted by transfusion, through breastfeeding, or sexually, but less effectively than HIV. The virus is associated with adult T-cell leukemia and a chronic progressive disease of the central nervous system. It was termed HTLV-1 – associated myelopathy or tropical spastic paraparesis, depending on the location where it was observed [39]. The causal relationship of HTLV-II and myelitis [11, 40] is not yet clear.
The classic course of this myelopathy is chronic, taking several years to develop, and bladder dysfunction may precede the onset of paraparesis [40]. The disease affects mainly the thoracic part of the spinal cord, but may also involve the cervical segment or both regions concurrently [39].
Imaging. The chronic course is manifested by atrophy of the lateral columns, following degeneration and demyelination of pyramidal, spinocerebellar, and spinothalamic tracts.
These lesions are associated with perivascular and parenchymal lymphocytic infiltration, proliferation of astrocytes, and fibrillary gliosis. Unlike VM and HIV, there seems to be an association between the presence of myelopathy and viral load in the spinal cord and CSF [11].
Less commonly, the disease develops within several months.
Imaging. In these more subacute cases, or alternatively during an initial inflammatory phase of chronic cases, extended lesions can be observed consisting of high signal intensity on T2WI with or without swelling [40]. The lesions can be found in the center of the spinal cord, bilaterally, or in the posterior columns.
When these lesions were surveyed [39], swelling and T2-lesions decreased and were followed by atrophy. Two out of eleven patients in the series of Umehara et al. [39] showed enhancement in the lateral columns, which was believed to reflect severity of inflammation. Periventricular and subcortical white matter lesions are present in 80 % of cases on brain MRI [40].
Prior to the development of antibiotics, syphilis was the most frequent cause of spinal disease, but occurred rarely in the absence of involvement at other sites of the nervous system.
Historically, tabes dorsalis has been viewed as the prototypical spinal disorder associated with syphilis [11], occurring on average 10–15 years after infection. It is characterized by “lancinating” or “lightning” pain, ataxia, loss of proprioception, trophic abnormalities, and autonomical dysfunction, most often attributed to selective degeneration of the dorsal spinal roots, the spinal root ganglia, and Wallerian degeneration of the posterior columns (Fig. 15.8). The pathogenesis of the lesions in the spinal roots is not well known, but chronic meningeal inflammation around the spinal roots is indicated. Unlike the cerebral cortex in “general paralysis of the insane,” there is no demonstrable inflammation in the spinal cord and Treponema pallidum is absent. Radiologists should be cautious: the differential diagnosis of a lesional pattern including the dorsal columns should embrace tabes dorsalis and be followed by a contrast enhanced study of the brain.