Gram-positive pathogens
Gram-negative pathogens
• Pneumococci
• Streptococci (S. pyogenes, S. faecalis)
• Staphylococci
• Listeria
• Meningococci (Neisseria meningitidis)
• Haemophilus influenzae
• E. coli
Acute exudative phase | Proliferative phase after 3–6 days of adequate treatment | Reparative phase after 10–14 days of adequate treatment |
• Purulent, cloudy appearance of CSF • Granulocytic pleocytosis with 1000–6000 cells/μL, less often > 15 000–20 000 cells/μL • High increase in protein levels to > 100–500 mg/dL, sometimes > 1000 mg/dL due to severe blood–CSF barrier dysfunction with QAlb > 20 × 10−3 • Occasionally, intrathecal IgA and/or IgM production detectable • Microscopic detection of bacteria • Antigen detection by latex agglutination test • Bacterial growth in CSF culture • Glucose ↓ (< 0.6 of serum level) • Lactate ↑ (> 3.5 mmol/L) • Lysozyme ↑ (> 1 mg/L) • In patients with unfavorable prognosis: – Very high cell count: > 10 000/μL – High lactate level – High bacterial count: > 106/mL CSF – Delayed CSF sterilization (> 36 h) after the start of antibiotic treatment | • Cell count: usually several hundred cells/μL • Polymorphic cell image, showing transformed lymphocytes, plasma cells, activated monocytes, and neutrophils • Protein, glucose, and lactate levels tend to normalize • No microscopic detection of bacteria, sterile CFS culture | • Cell count: < 50 cells/μL • Cytology nearly normalized • Protein, glucose, and lactate levels normalized |
Detection of pathogens. If bacterial meningitis is suspected on the basis of clinical examination, gram-stained preparations (Chap. 5, “Microscopic Detection of Pathogens”) are required in addition to normal differential cytology for detecting the pathogen (Table 10.1). If amebic infection is suspected, the native CSF should be screened for pathogens (e. g., in the counting chamber). Flagellated trophozoites are highly motile in a temperature-dependent way; it is therefore advisable to gently warm the CSF sample to body temperature prior to observation.
In immunosuppressed patients with clinically suspected bacterial meningitis, gram-stained preparations and CSF cultures are essential even when cell counts are normal.
Additional microbiological analysis. The following rapid diagnostic tests are used in addition to direct microscopic pathogen detection in gram-stained preparations and CSF culture, the results of which are not available for several days:
• Latex agglutination test: This is used for detecting bacterial antigens in CSF, and its results are available within a few minutes. However, the sensitivity is lower than that of CSF culture and roughly equivalent to that of microscopic detection. This method is used shortly after the start of antibiotic treatment, since it can still detect antigens at a time when pathogen growth in culture is no longer possible. In addition, this method allows speciesspecific identification. Latex agglutination tests are available for the following pathogens: Haemophilus influenzae, Streptococcus pneumoniae, group B streptococci, E. coli K1, Neisseria meningitidis (serotypes A, B, C, Y, W135).
• Limulus amebocyte lysate test: This test uses extracts of blood cells from the horseshoe crab, Limulus polyphemus, to detect minute amounts of bacterial endotoxin. However, the test is only suited to gram-negative pathogens.
• PCR: In principle, amplification of bacterial DNA by PCR can be used detect most pathogens of meningitis, but at present it is not employed in the diagnosis of acute bacterial meningitis. Studies have shown PCR detection of meningococcal DNA to have both a sensitivity and a specificity of about 90%; however, this test is usually not required for routine diagnosis.
Other laboratory parameters. If acute bacterial meningitis is suspected, a throat swab and at least one blood culture should be obtained before treatment with antibiotics is started. If there are skin lesions, additional wound swabbing or skin biopsy is recommended. If the patient has been exposed to rickettsia, a skin biopsy from the site of the arthropod bite is used to confirm the diagnosis of rickettsial infection.
Any further diagnostic laboratory tests depend on the patient’s history of exposure and associated symptoms.
Interpretation
Typical meningitis. The typical CSF findings in bacterial meningitis are summarized in Table 10.2 (see also Fig. 19.3a).
Follow-up lumbar puncture. In patients with acute bacterial meningitis, repeated CSF tests during the first few days are advisable, as with adequate treatment the CSF should become sterile within 24–36 hours (Table 10.2). If this does not happen, the antibiotic treatment must be changed. If the cell count is initially rather low, it will often increase during the first few days even if treatment is sufficient. As long as the bacterial count clearly decreases at the same time, or the CSF becomes sterile and the lactate concentration decreases, this rise in cellular immunity is regarded as positive for the course of healing. Increasing total protein concentrations may be due to impaired CSF circulation and may indicate the development of hydrocephalus or spinal abscesses.
Continued follow-up until CSF findings normalize, or until after treatment is complete, is not indicated, so long as no complications are seen in the clinical course. CSF cell counts after the end of treatment do not correlate with the risk of relapse.
Nonpurulent meningitis. In the nonpurulent form of bacterial meningitis, the typical CSF changes are absent as follows:
• When lumbar puncture is performed during the earliest (preneutrophilic) phase of bacterial sepsis.
• In patients punctured very late during sepsis with pancytopenia.
• In immunosuppressed patients.
• After splenectomy.
• In the presence of cerebral phlegmons and septic cerebral infarcts.
• When the patient has already been treated with antibiotics.
In nonpurulent bacterial meningitis, cell counts can be normal or slightly increased, and the blood–CSF barrier dysfunction is only moderate. The bacterial count is usually high.
Other laboratory parameters. There are usually signs of systemic inflammation, with pronounced leukocytosis, left shift, and a marked increase in C-reactive protein (CRP). However, these systemic signs may be absent in immunosuppressed persons. Electrolyte imbalances, particularly hypo- or hypernatremia, are suggestive of complicating hypophyseal dysfunction (symptom of inappropriate antidiuretic hormone, SIADH), while abnormal liver and pancreatic enzymes, increased urea and creatine or elevated creatine kinase suggest involvement of the respective organs.
Complications
Septic shock. Gram-negative bacterial meningitis is often complicated by symptoms of septic shock, including symptoms of disseminated intravascular coagulopathy with formation of multiple thrombi and a tendency to massive bleeding. Laboratory tests reveal decreases in thrombocytes, antithrombin III, and fibrinogen; they also show prolonged PTT, increased INR, and the presence of fibrinogen degradation products.
Septic sinus thrombosis. Septic thrombosis of the intracranial sinuses occurs as a complication of bacterial meningitis or purulent infection of the nasal sinuses. Changes in the CSF correspond to those of the underlying disease. In the case of bacterial meningitis, cell counts show a considerable increase in granulocytes which is accompanied by a sharp rise in total protein and decreased glucose/increased lactate levels. In cases of mastoiditis and transverse sinus thrombosis, the symptoms of meningeal irritation may be either absent or nonspecific, with mild, mostly mononuclear pleocytosis, a low granulocyte fraction, normal glucose values, and mild blood–CSF barrier dysfunction. Septic thrombosis of the cavernous sinus usually also leads to mild, nonspecific CSF changes.
Pitfalls in Septic Sinus Thrombosis
If septic sinus thrombosis is accompanied by subdural empyema, lumbar puncture is usually contraindicated because of the risk of herniation.
Chronic Meningitis
Etiology
The most important bacteria leading to a chronic course of meningitis are:
• Borrelia species.
• Treponema pallidum.
• Mycobacterium tuberculosis.
• Listeria monocytogenes.
Other bacteria (Brucella species, Pasteurella tularensis, and Leptospira, Nocardia, and Actinomyces species) are far less common. Parameningeal infections and subacute bacterial endocarditis can also lead to meningeal involvement.
Clinical Features
Bacteria may cause chronic meningitis which is defined by a set of typical symptoms:
• Headaches for more than 4 weeks.
• Involvement of cranial nerves.
• Vascular ischemic lesions.
• Hydrocephalus.
Diagnosis
On principle, headaches lasting more than 4 weeks and accompanied by subfebrile temperatures require lumbar puncture to exclude chronic meningitis. CSF changes are less characteristic in chronic bacterial meningitis than in the acute form and the differential diagnosis can include a wide spectrum of disease. Several lumbar punctures, additional microbiological tests, exposure history, and the analysis of systemic manifestations and concomitant symptoms are frequently required for a definite diagnosis.
Bacterial Endocarditis
Lumbar puncture is indicated in all patients with endocarditis who show central nervous system symptoms. However, the changes in the CSF vary considerably (Table 10.3).
Tuberculous Meningitis
Clinical Features
In this common form of cerebral tuberculosis, clinical symptoms of basal meningitis and the development of CSF circulation disturbances usually predominate (see also Fig. 19.3 b).
Diagnosis
Lumbar puncture is essential for diagnosis, although contraindications must of course be observed (caveat: watch out for obstructive hydrocephalus).
Interpretation
Macroscopic findings. The CSF may be slightly opaque to very cloudy, sometimes even xanthochromic. Spiderweblike clots due to spontaneous coagulation may be observed when a great deal of plasma is present as a consequence of severe blood–CSF barrier dysfunction. However, these clots are not pathognomonic of tuberculous meningitis.
Cell counts. Cell counts are slightly or moderately increased from 10 to about 1000 cells/μL, rarely more. They vary depending on the stage of the disease. The granulocyte fraction usually does not reach 50%, and eosinophilic granulocytes are frequently detected. Granulocytes may predominate early on, but usually there is mixed-cell pleocytosis with lymphocytes predominating and showing clear signs of activation. The B-cell fraction rises with the synthesis of immunoglobulins A and G, with IgA predominating. The lymphocyte and plasma cell fractions increase during treatment, and a subsiding pleocytosis usually persists over several months.
Lactate, glucose. CSF lactate and glucose levels depend on the acuteness of the disease:
• If the course is subacute, the glucose level is markedly lowered (CSF/serum glucose ratio < 0.3) and the lactate level raised.
• If the course is protracted, glucose and lactate levels may be only slightly pathological or may even be normal.
Total protein. In all patients with tuberculous meningitis, total protein is typically increased because of severe blood–CSF barrier dysfunction. Total protein levels are mostly between 2500 mg/L and 10 000 mg/L, sometimes even higher. The CSF/serum albumin quotient is frequently above 25. Very high values indicate disturbed intracranial or spinal CSF circulation. Usually, intrathecal synthesis of IgA (about 80%; Fig. 19.3 b) is detectable at an early stage; it is almost always present later in the course of the disease (Leary, 2000; Reiber et al., 2001). Intrathecal synthesis of IgG is found only in every second patient.
Other sets of findings. In tuberculous spondylitis, which is often accompanied by paraspinal abscesses, protein is usually very much increased as the result of disturbed CSF circulation. The cell count is only slightly increased nonspecifically as a sign of invasive meningitis. In cases of isolated tuberculoma, CSF may be normal in all respects.
Microbiological analysis. Since the number of mycobacteria in CSF is usually very low, microbiological analysis requires high sensitivity. None of the available methods is reliably highly sensitive and specific, so the microbiological test procedures have be combined and, if necessary, repeated.
• Direct microscopical pathogen detection on the basis of Ziehl-Neelsen staining (Chap. 5, “Microscopic Detection of Pathogens”) is successful only in 10–15% of cases, and even then only after repeated lumbar punctures.
• CSF culture requires at least 3–5 mL of CSF. Detection of mycobacteria in the culture is successful only in 30–50% of cases; usually, the results become unambiguous only after 3–8 weeks.
• Immunological tests to detect mycobacterial antigens and antibodies (ELISA, immunoblot) show high specificity but low sensitivity; they are not well established in clinical routine.
• Detection of mycobacterial DNA in CSF by means of PCR yields results within 24 hours; its sensitivity is rated from 50% to almost 100% (Lin et al., 1995; Fresquet-Wolf et al., 1998; Johansen et al., 2004).
• Determination of tuberculostearic acid (a constituent of the mycobacterial cell wall) by mass spectroscopy or gas chromatography also yields rapid results. The sensitivity of this method is 70–90%, with a specificity higher than 90% (Luef et al., 1992).
References
Fresquet-Wolf C, Haas J, Wildemann B, Storch-Hagenlocher B. Value of polymerase chain reaction (PCR) for diagnosis of tuberculoid meningitis. Nervenarzt 1998;69:502–506
Johansen IS, Lundgren B, Tabak F, et al. Improved sensitivity of nucleic acid amplification for rapid diagnosis of tuberculous meningitis. J Clin Microbiol 2004;42:3036–3040
Leary SM, McLean BN, Thompson EJ. Local synthesis of IgA in the cerebrospinal fluid of patients with neurological diseases. J Neurol 2000;247:609–615
Lin JJ, Harn HJ, Hsu YD, et al. Rapid diagnosis of tuberculous meningitis by polymerase chain reaction assay of cerebrospinal fluid. J Neurol 1995;242:147–152
Luef G, Allerberger F, Cheng A, et al. Diagnosis of tuberculous meningitis by detection of tuberculostearic acid in cerebrospinal fluid. Wien Klin Wochenschr 1992;104:322–324
Pfister HW. Bakterielle Infektionen. In: Brandt T, Dichgans J, Diener HC, eds. Therapie und Verlauf neurologischer Erkrankungen. Stuttgart: Kohlhammer; 2003
Reiber H, Peter JB. Cerebrospinal fluid analysis: disease-related data patterns and evaluation programs. J Neurol Sci 2001;184:101–122
Further Reading
Leary SM, McLean BN, Thompson EJ. Local synthesis of IgA in the cerebrospinal fluid of patients with neurological diseases. J Neurol 2000;247:609–615
Lehmitz R, Mix E, Zettl U. Entzündliche Erkrankungen des Nervensystems. In: Zettl UK, Lehmitz R, Mix E, eds. Klinische Liquordiagnostik. Berlin: Walter de Gruyter; 2003
Prange H. Infektionskrankheiten des ZNS. Weinheim: Chapman & Hall; 1995
Schmutzhard E. Entzündliche Erkrankungen des Nervensystems. Stuttgart: Thieme; 2000
Straussberg R, Harel L, Nussinovitch M, Amir J. Absolute neutrophil count in aseptic and bacterial meningitis related to time of lumbar puncture. Pediatr Neurol 2003;28:365–369
Abscesses
Brain Abscess
Definition
Cerebritis and brain abscess represent different stages of an infection by pathogens that can cause liquefaction of brain tissue.
Etiology
Dissemination of nearby infections, hematogenous metastatic invasion, open craniocerebral injuries, previous neurosurgical infections, and immunosuppression are important predisposing factors for cerebral abscesses and also determine the spectrum of pathogens. In addition to the entire spectrum of bacteria, abscesses of the brain can be caused by various fungi, protozoa, and helminths. Brain abscesses may be solitary or multiple.
Diagnosis
If a brain abscess is suspected, diagnostic imaging always has priority; CSF analysis then follows for identification of the pathogen.
Signs of systemic inflammation. Typical signs of systemic inflammation are of diagnostic value but may also be absent. Usually, they are very pronounced only in primary systemic infections with secondary brain abscess. Fever or subfebrile temperatures occur only in about 50% of cases. Leukocytosis is rarely severe, and up to 40% of patients with a cerebral abscess even have normal leukocyte counts. In about 75% of patients, the erythrocyte sedimentation rate (ESR) increases to over 40 mm/h, and CRP is usually moderately to strongly increased.
CSF findings. CSF findings vary considerably depending on the stage of the disease, localization of the brain abscess, and anatomical proximity to the subarachnoid space:
• Inflammatory reaction: The inflammatory reaction in CSF is most pronounced during the first 10 days of brain tissue infection; it is characterized by several hundreds of cells per microliter, granulocyte dominance, and moderate to severe blood–CSF barrier dysfunction. Intrathecal IgA synthesis is often observed. A similar set of findings also occurs at a later stage, once the abscess has perforated and found access to the subarachnoid space.
• Normal CSF: At the stage of encapsulation of the abscess, CSF may be normal in all respects.
• Pleocytosis: CSF shows mostly mild, nonspecific mixedcell pleocytosis with mononuclear cells predominating. The granulocyte fraction is rarely more than 50%. Most lymphocytes show only minor signs of activation, and macrophages are frequently detected. The albumin quotient (QAlb) is usually moderately increased up to 20 × 10−3, and the total protein up to 1500 mg/L. Local immune reactions take place only from the second week on, and intrathecal synthesis of IgA often predominates. Glucose levels are normal or slightly decreased.
Pathogen detection. The most important diagnostic step is collection of infectious material for culture and antibiogram. By using gram-stained CSF sediment and applying culture techniques correctly, pathogen detection is achieved in up to 80% of cases. If a brain abscess is suspected, anaerobic CSF cultures must always be used in addition to aerobic ones. If Toxoplasma abscess or tuberculous abscesses are suspected, specific diagnosis is possible by the following methods:
• PCR assay (this method has not been established for most pathogens).
• Determination of tuberculostearic acid (for suspected tuberculoma).
• Determination of antibodies and determination of intrathecal antibody synthesis (for suspected Toxoplasma abscesses).
In addition, an attempt should always be made to detect the pathogen by the analysis of extracerebral material (blood culture, puncture of a primary focus, other body fluids).
Spinal Epidural Abscess
Most abscesses of the spinal canal are epidural.
Etiology
Acute abscesses usually develop as a result of hematogenous dissemination, chronic abscesses usually by tissue invasion. The most common pathogens are gram-positive cocci.
Diagnosis
CSF findings. A raised CSF protein concentration is usually the predominant abnormality, with values reaching up to several thousand milligrams per liter because of impaired CSF circulation or CSF stasis. In addition, there is moderate mixed-cell pleocytosis. In the case of tissue invasion, dissemination of the infection leads to bacterial meningitis with a typical set of symptoms: granulocytosis, increased protein concentration, and decreased glucose with increased lactate concentration.
Pathogen detection. In cases of bacterial meningitis, pathogens are detected in gram-stained preparations and in CSF cultures. If the CSF findings point to a parameningeal infection, CSF cultures are positive only in 50–70% of cases and parallel blood cultures even less often. Rarely, anaerobic pathogens may cause spinal epidural abscesses, so both aerobic and anaerobic blood cultures are required.
CT-guided aspiration. A higher hit rate with close to 100% pathogen detection in both gram-stained preparations and cultures is achieved by CT-guided aspiration of the abscess. However, antibiotic treatment prior to diagnosis reduces the chance of pathogen detection to about 40%.
Ventriculitis
Etiology
Shunts. Modern neurology and neurosurgery often make use of ventricular catheters, internal or external drainage systems, and diagnostic intracranial monitoring systems. The implantation of foreign material increases the risk of infections. Shunt-associated ventriculitis is thus the most common form of ventriculitis. The most common pathogens are coagulase-negative staphylococci, enterococci, Klebsiella, and Pseudomonas species. In recent years, the number of patients with gram-negative ventriculitis has increased. Staphylococci are most commonly associated with grampositive ventriculitis (90% of cases).
Other causes. Other causes of ventriculitis are much less common. They are observed in fulminant cytomegalovirus encephalitis and in severe bacterial meningitis; such patients require intrathecal antibiotic therapy and the prognosis is poor.
Diagnosis
Lumbar puncture. When a ventricular shunt system is in place, most of the CSF produced in the ventricles is drained immediately after its production and because of this “reverse flow” hardly comes into contact with the lumbar CSF. CSF obtained by lumbar puncture is therefore often normal and shows the inflammation only at an advanced stage of spread.
If ventriculitis is suspected, the CSF should always be obtained from the ventricles under proper sterile conditions. If the distal limb of the shunt is infected, ventricular CSF is often normal. A search for signs of peripheral inflammation and repeated blood cultures are therefore essential diagnostic measures.
CSF findings. Changes in CSF vary and depend on the acuteness and the pathogen:
• Gram-negative ventriculitis: Test results frequently reveal granulocytic pleocytosis, highly increased protein levels, and decreased glucose/increased lactate levels.
• Gram-positive ventriculitis: Test results often show only mild inflammatory changes with moderate mixed-cell pleocytosis, moderate blood–CSF barrier dysfunction, and normal glucose and lactate levels.
The use of indwelling ventriculoperitoneal or ventriculoatrial shunts often leads to low-level, chronic inflammation with mild mixed-cell pleocytosis and normal glucose and lactate levels. More often the differential cytology shows eosinophilia. However, the same set of symptoms is also observed as an aseptic allergic reaction against the foreign material (Traynelis et al., 1988; Tung et al., 1991).
Pathogen detection. Pleocytosis associated with an indwelling shunt system should always prompt careful microbiological investigation of CSF cultures, repeated as may be necessary.
Correction of cell count. If symptoms of ventriculitis appear shortly after implantation of the shunt, the CSF is usually hemorrhagic. In this case, pleocytosis may also be reactive or caused by the admixture of blood, thus necessitating correction of the CSF cell count: per 1000 erythrocytes, one leukocyte is deducted from the total cell count. The following formula (Schmutzhard, 2000) is recommended for use:

Ideally, the value for this cell index is 1; values higher than 5.2 indicate an infection. A sudden increase by 4 or more within 24 hours suggests incipient ventriculitis. Other inflammatory markers in CSF are only detectable hours later, and systemic inflammatory markers appear later again. Ventriculitis associated with an overflow drain and open drainage system, is usually accompanied by bloody CSF, and the risk of nosocomial infection is high. In a patient with indwelling drainage, regular daily CSF examination is useful for timely recognition of any increase in cell count or cell index as early signs of infection—the more so since other variables, such as total protein content or glucose level, may no longer be of any use because of admixture of blood.
References
Schmutzhard E. Entzündliche Erkrankungen des Nervensystems. Stuttgart: Thieme; 2000
Traynelis VC, Powell RG, Kiss W, et al. Cerebrospinal fluid eosinophilia and sterile shunt malfunction. Neurosurgery 1988;23:645–649
Tung H, Raffel C, McComb JG. Ventricular cerebrospinal fluid eosinophilia in children with ventriculoperitoneal shunts. J Neurosurg 1991:541–544
Viral Infections of the Nervous System
Viral infections of the central nervous system mostly follow a systemic viral infection (Table 10.4). The viruses spread into the CNS either via the blood or along peripheral nerves. The clinical symptoms of many viral infections of the CNS and PNS are rarely sufficiently specific enough to permit a clinical diagnosis. Definite diagnosis of a viral disease of the nervous system relies on direct and/or indirect detection of pathogens.
Herpes Simplex Virus
Epidemiology
Depending on the disease, HSV-1 and HSV-2 are involved at different prevalences:
• Encephalitis: HSV encephalitis is the most common viral encephalitis with an incidence of 2–4 per million population per year. The causative pathogen is usually HSV-1; HSV-2 is causative in only 5–10% of cases.
• Meningitis: 5–10% of all viral meningitis cases are caused by HSV-2.
• Myelitis, radiculitis: HSV myelitis and radiculitis are rare and usually associated with HSV-2.
• Isolated facial paralysis: HSV viral infections may cause isolated facial paralysis, although this is under debate.
Pathogens
HSV-1 and HSV-2 are DNA viruses and exhibit marked cross-reactivity. They are distinguished by means of restriction enzyme analysis of their DNA. The majority of HSV-1 infections are asymptomatic, and 70–80% of the population are positive for HSV-1 by the age of 20.
Pitfalls in HSV Infection
HSV-1 and HSV-2 infections with a course showing the clinical picture of myelitis, radiculitis, or cranial mononeuritis do not present typical clinical findings. Only direct or indirect methods of pathogen detection will reveal the causal connection.
Diagnosis
Microbiological analysis. Detection of HSV DNA in CSF is the gold standard (sensitivity: 75–98%; specificity: 100%). It is possible to distinguish between HSV-1 infection and HSV-2 infection. PCR assay is positive during the early phase of the disease (< 9 days) and can detect HSV genomes up to 4 days after the start of virostatic treatment with acyclovir (Domingues et al., 1998). Detection of the virus in CSF cells is possible by in situ hybridization; its sensitivity is about 70%. However, this requires CSF sediments of high quality—something rarely achieved in practice. Isolating the virus from brain tissue has a high diagnostic hit rate but is of little practical significance. After PCR, detection of intrathecal synthesis of anti-HSV immunoglobulins (antibody index; Chap. 5, “Antibody Index”) is the second-choice method for emergency diagnosis, since positive results for virusspecific antibody production can not be expected earlier than 1–2 weeks after the onset of symptoms. Once the infection has been treated, or if the disease takes a prolonged course, detection of specific antibodies is the preferred diagnostic method. It should be remembered, however, that there is cross-reactivity with varicella-zoster virus (see Table 19.3) and that HSV-specific antibody synthesis may also be observed during a polyspecific immune response (MRZ reaction) in autoimmune diseases. In patients with isolated myelitis in particular, considerable problems in differential diagnosis may occur. On the other hand, in those with isolated facial paralysis or radiculitis, such as Elsberg syndrome, detection of specific viral antibodies is the only diagnostic method possible.
CSF analysis. In 20–30% of cases no CSF changes are seen during the acute phase of isolated encephalitis. During follow-up analysis, pathological changes may be found as the result of secondary meningeal dissemination (Fig. 19.4). Cell counts are 10–500 cells/μL, and the cells are usually lymphocytic; rarely, when puncture is carried out early, there are mixed cells with granulocytes dominating. Erythrophages and hemosiderophages are a sign of hemorrhagic encephalitis and are not uncommon. Total protein levels are between 500 and 800 mg/L, and lactate levels are slightly to moderately increased (< 3.4 mmol/L). About 1 week after the onset of disease, a humoral immune response occurs. Intrathecal IgG production is common and may be accompanied by local IgA and IgM synthesis.
Neuroimaging. About 36–48 hours after the start of neurological symptoms, MRI typically shows unilateral changes (temporopolar, basal, and frontobasal). These findings are characteristic and often help to determine the diagnosis—especially when no changes in the CSF are seen.
Varicella-Zoster Virus
Pathogen
Varicella-zoster virus (VZV) is the etiologic agent of chickenpox and herpes zoster. The infection is ubiquitous and is transmitted by droplets. Most humans have been infected by the age of 20, and 30–60% of these infections become clinically manifest. VZV is a DNA virus belonging to the Herpesviridae family.
Clinical Features
VZV infection during childhood is harmless, but in later life it can lead to serious neurological complications, such as meningitis, meningoencephalitis, cranial neuritis, myelitis, ganglionitis, radiculitis, vasculitis, or postzoster neuralgia.
Diagnosis
Laboratory analysis. PCR determination of VZV DNA in CSF is the method of choice during the acute phase (sensitivity: up to 95%). In 50% of cases of VZV meningitis, a pathogenspecific humoral immune reaction can be observed after only 6 days (Felgenhauer et al., 1992). During the course of the disease, the sensitivity of the VZV antibody index increases to almost 100%. In principle, a local immune reaction can be detected in all VZV-induced diseases of the nervous system.
Pitfalls in VZV Diagnosis
As in HSV infection, the interpretation of findings is hampered both by the cross-antigenicity between HSV and VZV and by the need to distinguish between VZV identification and positive results due to a MRZ reaction in chronic inflammatory disease. An isolated VZV antibody index must therefore be taken with caution and considered only in the context of all CSF and clinical findings.
CSF analysis. CSF findings depend on the neurological manifestation:
• Cell counts in isolated VZV meningitis vary between 30 and 300 cells/μL.
• Cell counts in VZV ganglionitis may be normal (20%) or only slightly increased (30%). The cells are lymphocytic, and total protein is increased to 500–1000 mg/L.
• Barrier dysfunction is common in VZV meningitis (about 90%), whereas in VZV ganglionitis normal values are almost always observed (Fig. 5.26a).
• Local immune reaction is weak; the quotient diagram shows IgG synthesis in about 15% of cases, and IgM synthesis is rarely observed. In VZV ganglionitis, the VZV antibody index (IgG class) is increased without exception (see Chap. 19 “Evaluation”).
Tick-borne Encephalitis
Epidemiology, Pathogen
The etiological agent of tick-borne encephalitis (TBE) is an RNA virus of the family Flaviviridae. Two subtypes are known: the Central European encephalitis (CEE) virus and the Russian spring–summer encephalitis (RSSE) virus. The viruses are transmitted by ticks and, in Eastern Europe, occasionally also by unpasteurized sheep or goat milk. The infection rate of ticks ranges between 0.1% and 5%. In Germany, over 1600 persons fell ill between 1991 and 2001. The main distribution area in Germany is south of the river Main. Besides Germany, the disease is endemic in Austria and much of Europe as well as the former Soviet Union.
Clinical Features
Clinical symptoms after infection with CEE virus occur in about 50% of cases. In the remaining cases, the infection is asymptomatic. Among the neurological complications that can occur are isolated meningitis (50%), encephalitis (40%), and myelitis (10%). In most patients, the neurological symptoms are not particularly characteristic; however, atrophic paralysis may occur as a sign of anterior horn disease, or extrapyramidal-motor symptoms may occur if the basal ganglia are affected. These signs may then point to CEE infection. Patients suffer conspicuously from a severely impaired general state of health with fever, headaches, and fatigue.
Diagnosis
Microbiological analysis. In the prodromal phase, clarification of the etiology is possible only by identifying the antigen or pathogen (PCR, culture) in the serum; once neurological symptoms appear, detection is possible only in the CSF. However, the diagnostic value of direct pathogen detection is insignificant and currently restricted to solving specific problems.
The diagnosis of CEE is based on detection by ELISA of pathogen-specific antibodies (IgM and IgG) in serum in combination with inflammatory CSF symptoms.
About 7–10 days after infection, IgM antibodies can be detected in the blood, indicating a recent infection; IgG antibodies follow in the second week. Simultaneous detection of IgM and IgG, or a 4-fold titer increase of specific IgG in serum, is diagnostic. Absence of the IgM response is rarely reported. IgM antibodies disappear after weeks or months, whereas IgG antibodies persist. In endemic regions in Europe, anti-CEE antibodies are present in 14–42% of the population. Cross-reactions with other flaviviruses may occur (e. g., after yellow fever vaccination). Detection of intrathecal synthesis of pathogen-specific IgG antibodies also confirms the diagnosis. A pathological antibody index for CEE IgG is found in 90% of cases (and for IgM in 60%) 2 weeks after the first symptoms occurred. Calculation of the antibody index is of special importance whenever CEE is suspected in vaccinated persons in whom immunization is not yet complete. A humoral CEE-specific immune response in CSF is expected only after CEE infection; it does not occur after vaccination.
CSF analysis. CSF is normal during the prodromal stage. Pleocytosis (30–1500 cells/μL), increased protein levels (250–2200 mg/L), and barrier dysfunction (70%) are observed at the beginning of the second phase of the disease. Frequently, the initial cytology shows granulocytes; later, lymphocytes become dominant. In patients with neuroborreliosis, by contrast, the first lumbar puncture reveals markedly activated lymphocytes and plasma cells. Humoral immune reactions are regularly observed (IgG 25%, IgM 50%, IgA 12%); the most common reaction is a two-class response with local synthesis of IgM and IgG.
West Nile Virus
Epidemiology
West Nile virus (WNV) was first isolated and identified in 1937 from an infected person in the West Nile district of Uganda. The virus is widely distributed in Africa, Europe, Australia, and Asia. Since 1999 it has spread rapidly through the Western hemisphere including the USA, Canada, Mexico, and the Caribbean, and into parts of Central and South America. Before 1994, outbreaks of WNV were sporadic and mainly associated with mild febrile illness. Since 1994 outbreaks have occurred with a high incidence of severe human disease, particularly affecting the nervous system. WNV is a mosquito-transmitted flavivirus. Birds are involved in the cycle of transmission as amplifying hosts. Humans and horses are considered accidental dead-end hosts. New modes of transmission through blood donations, organ transplants, and the intrauterine route have been reported. It is unclear whether the apparent change in disease severity and frequency is due to differences in the circulating virulence or to changes in other predisposing chronic conditions in the affected population.
Virology
WNV is a single-stranded RNA virus of the family Flaviviridae, genus Flavivirus. WNV is a member of the Japanese encephalitis virus serocomplex (e. g., Japanese encephalitis, St. Louis encephalitis). The virus can be divided genetically into two lineages. Only viruses of lineage 1 have been definitely associated with human disease. The WNV responsible for the 1999 outbreak of New York City was a lineage 1 virus that circulated in Israel from 1997 to 2000, suggesting viral import into North America.
Clinical Features
The incubation period of WNV ranges from 3 to 14 days. One in five infected persons develops mild febrile illness, often accompanied by malaise, myalgia, nausea, and vomiting; one in 150 persons develops meningitis, encephalitis, or both. Rare neurological presentation other than encephalitis or meningitis includes ataxia, complete flaccid paralysis, extrapyramidal signs, polyradiculitis, or myelitis. Case fatality rates among hospitalized patients have ranged from 4% to 12%. Advanced age is the most important risk factor for death. Long-term morbidity is high with persistent fatigue in 67%, difficulty in walking in 49%, and memory loss in 50% of surviving patients 1 year after discharge from hospital. Treatment for WNV infection is symptomatic.
Diagnosis
Diagnosis rests on the presence of WNV-infected birds, a high index of clinical suspicion, and the results of specific laboratory tests.
Laboratory findings. Total leukocyte counts in peripheral blood are mostly normal or elevated. Examination of the CSF shows pleocytosis with leukocyte counts ranging from 0 to 1782 cells/μL, usually with a predominance of lymphocytes. Protein levels are universally elevated (51–899 mg/dL), and glucose levels normal. Computed tomography of the brain usually shows no evidence of acute disease. In approximately one-third of patients, MRI reveals enhancement of the leptomeninges, the periventricular areas, or both.
Microbiological findings. The most effective diagnostic method is detection of IgM antibody to WNV in serum or CSF, with the IgM antibody-capture ELISA. Within 8 days of symptom onset 90% of serum samples are positive for IgM antibody.
Two points must be taken into account in interpreting the serological tests. A close antigenic relationship exists among the flaviviruses. For this reason, persons recently vaccinated with yellow fever or Japanese encephalitis vaccines or those recently infected with a related flavivirus may have positive results on IgM antibody tests for WNV. The plaque reduction neutralization test can be used to distinguish false-positive results on IgM antibody-capture ELISA. The plaque reduction neutralization test may also help distinguish serological cross-reactions among the flaviviruses.
Secondly, because most infected persons are asymptomatic, and because IgM antibody may persist for 6 months or longer, people in endemic areas may have persistent IgM antibody from a previous infection that is unrelated to their current clinical illness. An increase in virus-specific antibody titers in serum specimens from persons with acute disease confirms acute infection. Another possibility is to isolate WNV or detect viral antigen or nucleic acid in CSF, tissue, blood, or other body fluids. Although positive tests are diagnostic, low sensitivities preclude their routine use as screening tests: results on nucleic acid amplification testing have been positive in only up to 55% of samples of CSF and 10% of serum samples.
Cytomegalovirus
Pathogen
The cytomegalovirus (CMV) belongs to the family of Herpesviridae. Infection may occur at any age, even during embryonic development, and persists in endothelial cells, with occasional reactivations. In Europe and North America, 0.1–0.5% of all newborns are considered to be already infected with CMV; in old age, antibody prevalence is 70–80%. After birth, the manifestation index of CMV infection in immunocompetent individuals is below 1%. In persons with immunodeficiency or immunosuppression, primary or reactivated CMV infections lead to severe complications.
Clinical Features
CMV-induced retinitis, colitis, or encephalitis occurs in about 40% of patients with AIDS. Since the introduction of highly active anti-retroviral therapy, the prevalence has dramatically declined. The neurological spectrum includes diffuse encephalitis, ventriculoencephalitis, myeloradiculitis or polyradiculitis, mononeuritis multiplex, and isolated optic nerve neuritis.
Diagnosis
CSF shows granulocytic or mixed-cell pleocytosis (about 150 cells/μL) and a variable increase in total protein. Direct pathogen detection in CSF is possible by PCR (sensitivity, 86–95%; specificity, 87–94%). With sensitive PCR protocols that facilitate the detection of single viral genomes, quantitative determination of the viral load can be used to distinguish latent from active infections (Wildemann et al., 1998). Determination of CMV pp67 late gene transcripts can serve as an additional test for viral replication in the nervous system (specificity, 100%; sensitivity, 86–94%). Detecting the pp65 antigen in serum as a sign of active replication is also helpful. Indirect pathogen detection by determination of IgG and IgM antibodies in CSF and serum is rarely diagnostically helpful in immunosuppressed patients. In immunocompetent individuals, detection of IgM or an increase in IgG titer in serum suggests recent infection. ELISA is the method of choice. In immunosuppressed patients, a threeclass humoral immune response in the CSF is observed (see Chap. 19, “Evaluation”).
Human Immunodeficiency Virus
Pathogen
The human immunodeficiency virus (HIV) is the etiologic agent of acquired immunodeficiency syndrome (AIDS). It is an RNA virus and a member of the family of Retroviridae. There are two serotypes: HIV-1 (subtypes A–H, M, O) and HIV-2. The virus predominantly infects CD 4-positive T lymphocytes, macrophages, and monocytes, less often B lymphocytes, astrocytes, and endothelial cells. The infection causes a steady decline in the number of CD 4-positive lymphocytes and an escalating disturbance of cellular immunity. As a result, opportunistic diseases develop caused by viruses, fungi, and mycobacteria. Nonspecific polyclonal activation of B cells is observed early in the course, and this also disturbs the specific immune defense. This immune dysregulation causes autoimmune reactions, which in their turn hamper laboratory analyses by producing irrelevant antibodies. In about 10% of cases, neurological complications are the first manifestation of the infection. Prior to introduction of highly active anti-retroviral therapy, the nervous system was affected in more than 50% of adults and in 90% of children in the advanced stage of the disease. Thanks to combination therapies, the prevalence of opportunistic infections has declined significantly. So far, however, this has had little effect on the frequency of neurological diseases directly associated with HIV.
Clinical Features
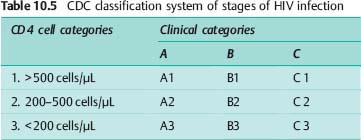
Since 1993, the stages of HIV infection have been categorized according to the classification system of the United States Centers for Disease Control and Prevention (CDC) (Table 10.5). The CDC system combines clinical categories A, B, and C (Table 10.6) with laboratory categories 1, 2, and 3, which are based on CD 4 cell count.
The type and frequency of neurological complications depend on the stage of the infection (Table 10.6). A distinction is made between HIV-associated neurological manifestations and those caused by opportunistic infections. Opportunistic infections usually occur only at stage C, when the CD 4 cell count is below 150 cells/μL. The HIV infection may affect the entire nervous system, causing various syndromes:
• HIV meningitis (stages A/B).
• HIV myopathy (stages A–C).
• HIV neuropathy (stages B/C).
• HIV myelopathy (stage C).
• HIV encephalopathy (stage C).
The clinical symptoms depend on the neuroanatomical structures affected; for example, HIV encephalopathy is characterized by neuropsychiatric deficits (memory problems, poor concentration, apathy) and motor deficits (impaired fine motor skills, extrapyramidal motor symptoms, cerebellar symptoms).
Diagnosis
Laboratory analysis. HIV antibodies can be detected 4–12 weeks after infection. The most commonly used screening test is ELISA. The following three methods are used for confirmation:
• Immunoblot.
• Radioimmune precipitation assay.
• Indirect immunofluorescence test.
A positive screening test should always be confirmed. HIV antigen tests are available; however, they are indicated for early diagnosis only, since they may become positive 1–2 – weeks earlier. Isolation of the virus from EDTA blood, plasma, or isolated lymphocytes is possible; however, it is not only time-consuming but also labor- and cost-intensive.
Molecular genetic methods have become the established approach to determining plasma viral load. They are well suited to monitoring both disease and treatment.
CSF analysis. Changes in the CSF appear at an early stage in 40–80% of asymptomatic HIV-infected patients. Characteristic findings are mild lymphocytic pleocytosis with isolated plasma cells, predominantly normal barrier function, and the presence of oligoclonal bands (up to 70% of cases) (Fig. 19.4 c). Local synthesis of IgA and IgM does not take place. The CSF syndrome in clinically apparent HIV meningitis is quantitatively different from that in asymptomatic HIV infection, but qualitatively it is the same. Intrathecal synthesis of HIV-specific antibodies is frequently seen in both neurologically asymptomatic patients and those with neurological manifestations, increasingly so in the later disease stages. The HIV antibody index is positive in more than 80% of patients with manifest AIDS (Elovaara et al., 1988; Luer et al., 1988). At the late stage of the disease (AIDS), the extent of intrathecal humoral and cellular immune reactions regresses. If, during the course of HIV infection—particularly in the later stages—CSF analysis shows intrathecal synthesis of IgG, IgA, and/or IgM in combination with activated B lymphocytes, it is highly likely that an additional opportunistic infection of the nervous system is present (Fig. 19.4 c). Opportunistic infections include viral infections (e. g., CMV, HSV, and VZV), toxoplasmosis, fungal infections, and neurotuberculosis. Because the immune reaction is already severely disturbed, molecular diagnostic methods are preferred.
In all stages of the disease, pathogen identification involves detection of HIV-specific genome sequences by PCR. It is possible to measure the viral load in the CSF; in individual cases of HIV-associated CNS manifestations this can be done to assess the effectiveness of anti-retroviral therapy; indirectly, it can also point to resistant mutants of the virus. It is noteworthy that opportunistic infections can also lead to an increased viral load in the CSF on the basis of existing CSF pleocytosis.
Infection of the Nervous System by Fungi and Other Opportunistic Pathogens
Toxoplasmosis
Epidemiology
Latent infection. Cerebral toxoplasmosis generally results from reactivation of a latent infection. The prevalence of latent infections is high. On average, about every third person is seropositive by the age of 30, and 70% are seropositive when 60–65 years old.
AIDS. Severe CNS infections occur almost exclusively in immunosuppressed patients. At the beginning of the AIDS pandemic, toxoplasmosis was the most common cause of focal neurological deficits in AIDS patients—and still is in African countries. The disease manifestation depends on the immune status of the patient. The introduction of highly active anti-retroviral therapy has significantly reduced the incidence of cerebral toxoplasmosis.
Congenital toxoplasmosis. Congenital toxoplasmosis is transmitted through the placenta. This requires an acute or reactivated infection in the mother. If the infection is recent, the risk of congenital disease is 30–50%. Infections during the first trimester are usually severe, whereas in the third trimester they remain often asymptomatic.
Pathogen
Toxoplasma gondii is an obligatory intracellular parasite; its sexual form develops in cats. In humans, infection is usually through eating raw or undercooked meat, especially pork. Most infections are acquired postnatally; they are clinically silent or associated with flu-like symptoms. After penetration of the intestinal wall, there is hematogenous dissemination into the muscles and the CNS, where in most immunocompetent persons asymptomatic cysts develop. If the cellular immune response is impaired, tachyzoites are released which then infect neighboring neurons and astrocytes, leading to formation of central necrotizing granulomatous foci.
Diagnosis
Laboratory analysis. Definite diagnosis is by direct detection of parasites in biopsy material (e. g., brain tissue). The methods used are classical staining (e. g., HE) or immunohistochemical procedures. Animal tests and tissue culture are also possible. Microscopical detection in CSF is rarely successful. Molecular diagnostic methods can be used on tissue or CSF. The sensitivity of PCR using CSF is about 50%, and the specificity is 100% (Novati et al., 1994; Cingolani et al., 1996). Serological tests using IFT or ELISA are almost always positive in cerebral toxoplasmosis (Fig. 19.4 c); only 3–6% of patients show negative results.
Since the infection is highly endemic in the general population, a positive serological result is of limited diagnostic value.
In immunosuppressed patients, a rise in serological titers is often absent. IgM is usually not detected. Nonspecific parameters in the CSF are mostly normal. Rarely, lymphocytic pleocytosis of up to 1000 cells/μL is seen; barrier dysfunction or a local immune response are more frequent. In up to 50% of patients, a specific intrathecal immune response against Toxoplasma gondii is detected (Fig. 19.4 c) (Borges and Figueiredo, 2004). In patients with MS the specificity of intrathecal antibody synthesis is restricted, since in 10% of them the Toxoplasma antibody index is increased as a result of polyspecific immune reactions.
Neuroimaging. MRI shows usually multiple contrast-enhancing ring-shaped lesions with perifocal edema at the corticomedullary junction or in the basal ganglia. Some 25–40% of lesions are solitary, and 5–20% of them do not enhance on administration of contrast medium.
Differential Diagnosis
The most important differential diagnosis in HIV patients is CNS lymphoma (Fig. 19.4 d). Other possible causes include HSV encephalitis, syphilis, EBV infection, CMV infection, fungal encephalitis, bacterial brain abscess, and progressive multifocal leukoencephalopathy. Empirical antibiotic treatment for 2 weeks is recommended before considering brain biopsy.
Cryptococcosis
Pathogen
Cryptococcosis is the most common mycosis selectively affecting the central nervous system. The yeast-like fungus is characterized by budding cells (4–6 μm) surrounded by a thick mucous capsule. The disease affects primarily individuals with a defective T-cell-mediated immune defense, such as patients with AIDS (about 5%, up to 33% in endemic regions) or cancer, and patients who have undergone solid-organ transplantation or are under long-term corticosteroid treatment or chemotherapy. Infection occurs by inhalation of cryptococci from bird feces. Depending on the immune defenses, the infection either remains restricted to the lungs or is disseminated through the blood to the brain and spinal cord.
Clinical Features
Clinical findings include acute, subacute, and chronic meningitis, rarely meningoencephalitis. First symptoms are bitemporal headache, initially intermittent and later persistent, followed by nausea and vomiting. Signs of intracranial pressure, basal cranial nerve deficits, blindness, and deafness may occur later. Without treatment, the disease is fatal within weeks or a few months.
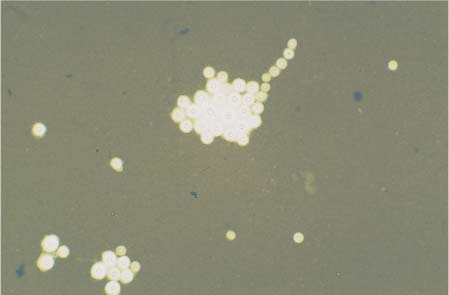
Diagnosis
Laboratory diagnosis is by direct microscopic detection of the pathogen in CSF using India ink preparations (Fig. 10.1). The yeast fungus must be distinguished from erythrocytes, granulocytes, and sebum. The fungus measures 4–6 μm and has a polysaccharide capsule of 1–30 μm. The India ink preparation is positive in 60–70% of non-AIDS patients with cryptococci, and in more than 90% of AIDS patients with cryptococci. If the findings are unclear or negative, detection by culture is still the gold standard. Detection of cryptococcal antigen using the latex agglutination test is also diagnostic. In non-AIDS patients, the CSF titer is below 1:1000; in AIDS patients it is above 1:1000. Sensitivity is lower for serum. Repeated testing increases the diagnostic value of the findings. In patients with CNS cryptococcomas, pathogen detection in the CSF is mostly negative. Antibody detection is of limited diagnostic value because these antibodies also occur in healthy persons. Cell counts may be normal or slightly increased, up to 300 cells/μL. Cytology shows a mixed-cell pattern dominated by lymphocytes, or only lymphocytes and plasma cells; total protein may be markedly increased up to 2500 mg/L. The immunoglobulin responses may be of the two-class or three-class type. Lactate levels of up to 13 mmol/L have been reported.
Candidiasis
Epidemiology
Candidiasis occurs predominantly under conditions of immune suppression, e. g., in patients with AIDS or those under long-term immunosuppressive treatment (such as after organ transplantation), cancer patients, premature babies with low birth weight, and patients undergoing long-term intensive therapy. Also at risk are patients who have undergone neurosurgical intervention, particularly shunt placement, or those patients with central venous catheters.
Pathogens
CNS candidiasis is usually caused by Candida albicans, less often by other Candida species (C. glabrata, C. tropicalis, or C. lusitania). The yeast-like fungi are commonly found as normal commensal flora, at least transiently, in the oral cavity and gastrointestinal tract, on the skin, and in the vagina. Characteristic are their round or oval budding cells (4–8 μm) and the formation of pseudomycelia from elongated cells. Involvement of the CNS is usually the result of hematogenous colonization, starting from an infection of other organs, particularly the gastrointestinal tact. Histopathology often reveals multiple small (< 2 mm) subcortical microabscesses. Meningitis and vasculitis are observed less often.
Fig. 10.1 India ink preparation of Cryptococcus neoformans.
Clinical Features
CNS candidiasis does not show a typical clinical picture. Chronic encephalitis with impaired consciousness, meningeal irritation, and focal deficits are common. The course may be acute (as in sepsis) or chronic.
Diagnosis
The diagnosis is made on the basis of CSF cultures and antigen detection in CSF. The disadvantage of antigen detection methods is their low specificity. Microscopical detection is successful in about 40% of cases, but the number of yeast cells is often below the detection limit for microscopy. Preanalytical requirements include the use of hypertonic transport media. If detection in body fluids is negative, biopsy of a CNS granuloma or cysts may yield positive culture results. Nonspecific CSF variables are normal or similar to those in bacterial meningitis. Detection of Candida antibodies in the serum is feasible but of no decisive diagnostic value.
At present, no suitable method exists that will distinguish with adequate probability between invasive candidiasis, superficial mucosal candidiasis, and Candida colonization.
Reduced antibody production in immunosuppressed patients further limits the diagnostic value of the tests. A diagnosis of invasive disease can be regarded as confirmed when any of the following criteria are met:
• Repeated detection in blood cultures obtained on different days.
• Detection in tissue biopsies by culture or histology.
• Detection in fluids that are normally sterile (e. g., CSF, synovial fluid), by either microscopy or culture.
Cell counts in the CSF of patients with candidiasis are usually 30–300 cells/μL. The cytology shows lymphocytes and granulocytes. Total protein levels are usually markedly increased to about 1500 mg/L. Lactate levels are always markedly increased. Intrathecal IgA synthesis, sometimes accompanied by IgG synthesis, is observed. In patients with abscess formation or granuloma distant from the meninges, CSF is often normal.
Aspergillosis
Pathogens
Molds of the genus Aspergillus are ubiquitous. They are found in great abundance in composted soil. In hospitals, construction or renovation work and contaminated air conditioning systems are considered the main sources of Aspergillus, and the main means of its dissemination. The most relevant species in human medicine is Aspergillus fumigatus, followed by the less frequent A. niger, A. flavus, A. terreus, and A. nidulans. Infections are nosocomial and affect immunocompromised patients, for example, after organ transplantation. Primary pulmonary manifestation occurs after inhalation of the pathogen. Hematogenous dissemination leads to solitary or multiple brain abscesses (47%), granuloma (35%), and, less often, to meningitis or ventriculitis (18%). The course is subacute or chronic. Mycotic aneurysms or thrombotic occlusions with secondary infarction may result from vascular involvement. Isolated meningitis in immunocompetent patients is rare.
Clinical Features
Clinical manifestations include personality changes, epileptic seizures, focal neurological deficits, and signs of meningitis. Concomitant pneumonia as a primary symptom may be indicative.
Diagnosis
Hyphae and the characteristic conidiophores are detected in both native preparations and sections of tissue samples. Aspergillus species grow on Sabouraud agar and other culture media within 2–7 days. Blood and CSF cultures are usually negative, even in cases of disseminated aspergillosis. Altogether, detection by microscopy and culture techniques as applied to body fluids is time-consuming and only rarely successful. Proof of CNS aspergillosis is best obtained from biopsy material, such as brain abscesses. Serological detection of the galactomannan antigen seems to be more sensitive than culture methods. The method used is a latex agglutination test. Its diagnostic sensitivity is about 50% and its specificity 60–100%. Repeated serum and urine analyses are recommended. Usually, positive reactions are observed only during the final stage. False-positive reactions due to other molds are possible. Antibody detection by means of ELISA systems is feasible, but the diagnostic sensitivity and specificity are low. Increased titers are indicative but are often absent because of underlying immunosuppression. CSF often shows moderate mixed-cell pleocytosis with eosinophilic granulocytes and increased protein levels. Cytological signs of hemorrhage (erythrophages, siderophages) are occasionally visible in the CSF and suggest possible concomitant vasculitis. Where there is meningeal involvement, cell counts are increased to 50–1000 cells/μL, with granulocytes being the dominant cell type. Total protein may be markedly increased up to 4000 mg/L. During the course of the disease, there is intrathecal immunoglobulin synthesis with IgA dominance and moderately increased lactate levels.
Neuroborreliosis
Epidemiology
Borreliosis, or Lyme disease, is the most common tick-borne zoonosis in Europe and North America. In Germany the estimated incidence is 60 000 new cases per year.
Pathogens
Genospecies. In Europe, there are three pathogenic genospecies: Borrelia burgdorferi sensu stricto, B. garinii, and B. afzelii. Using the OspA/OspC serotyping system, these three genospecies can be divided into 7 OspA serotypes and 13 OspC serotypes. Only the genospecies B. burgdorferi sensu stricto occurs in North America. B. burgdorferi is a gram-negative spiral bacterium of 10–30 μm in length and 0.2–0.25 μm in diameter.
Protein bands. More than 30 different protein bands can be identified by SDS gel electrophoresis (Fig. 4.12; Chap. 4, “Electrophoretic Methods with Immunodetection,” Western blot). Independently of their genospecies, Borrelia species possess two main protein components with constant molecular weights: p41 or flagellin (41 kDa) and HSP60 (60 kDa). Flagellin has cross-reacting as well as serotypespecific epitopes, whereas the heat shock protein HSP60 shows high cross-reactivity with other bacteria. Three other characteristic bands represent outer surface proteins with variable molecular weights: OspA (31–33 kDa), OspB (34–36 kDa), and OspC (20–23 kDa). Other well-characterized membrane-bound Borrelia antigens (mostly encoded by plasmids) include p83/100, Oms66 (p66), BmpA (p39), p18, OspD, OspE, and OspF. More recently, recombinant variable surface antigen (VlsE) has become important for Borrelia diagnosis.
Disease Stages
Onset. Animal experimental data show that infection from the bite of an infected tick depends on the tick’s sucking time, developing in between 7% (36 hours) and 75% of those bitten (48 hours). The infection is clinically silent in 95% of infected persons, i. e., it resolves spontaneously and is sometimes accompanied by seroconversion. In the remaining 5%, clinical symptoms appear, usually on the skin. The incubation period is usually 5–48 days (12 days on average). In over 80% of cases, the disease begins with erythema migrans as the confirmatory skin manifestation of stage 1.
Course. Borreliosis is a multisystem disease which evolves in stages and may affect the skin and nervous system. Clinically, the stages are divided according to the time of infection or, in case of a known tick bite, according to the duration of the disease (Table 10.7). Borreliosis may run through all three stages, skip stages, or become symptomatic at any stage.
Stage 1. About 20% of patients complain of nonspecific, generalized symptoms with muscle and joint pain as signs of the hematogenous dissemination that has already taken place. One in every seven people with the disease goes through stage 1 without symptoms. Without antibiotic treatment, the infection resolves without sequelae in over 90% of cases.
Stage 2. This stage begins 2–10 weeks after infection. The clinical course is biphasic in every second affected person. After spontaneous disappearance of the erythema, secondary symptoms may involve the peripheral nervous system, meninges, joints, heart, and eyes. At this stage, lymphocytic meningoradiculitis is clinically prominent. Without antibiotic treatment, there is spontaneous remission in over 99%, and complete recovery occurs in about 30% of cases.
Stage 3. Unlike stages 1 and 2, stage 3 is characterized by chronic destructive disease of the skin, joints, and nervous system without any tendency to spontaneous recovery. By definition, stage 3 is reached when the disease has progressed over the course of 6 months. This classification is based on the observation that patients in stage 2 usually show spontaneous recovery within 6 months. If they do not do so, they can no longer be expected to recover without treatment.
Reports of cases showing clinically apparent symptoms in all three stages are very rare. The frequencies of different organ manifestations are shown in Table 10.8.
Borrelia burgdorferi may affect the entire neuroaxis, leading to a wide spectrum of neurological manifestations: mononeuritis or polyneuritis, lymphocytic meningitis, myositis, chorioretinitis, meningoradiculitis, transverse myelitis, myeloradiculitis, focal or generalized encephalitis with extrapyramidal motor symptoms, cerebellar ataxia, hemiparesis, exogenous psychosis, epileptic seizures, cerebral vasculitis, and progressive encephalomyelitis (Table 10.8). In stage 2 meningoradiculitis is the most common manifestation, occurring in 74–86% of cases.
Cranial nerves are rarely affected—if they are, then predominantly during childhood and adolescence, and usually the facial nerve. Acute meningomyelitis and meningoencephalitis each occur in about 5% of cases. The second typical syndrome during childhood and adolescence is isolated meningitis; it is difficult to detect because it is frequently seronegative at the beginning (75%). Like neurosyphilis, there is a subacute cerebrovascular form of neuroborreliosis, although it is far less common. The progressive form of encephalomyelitis occurs at stage 3 and is rare. Cardinal symptoms include spastic paraparesis, spinal or cerebellar ataxia, organic psychosis, hemiparesis, and extrapyramidal motor symptoms. Concomitant damage of peripheral nerve structures may occur, in particular with involvement of the optic and vestibulocochlear nerves. The course is chronic and progressive, rarely with exacerbation episodes. Chronic polyneuritis has been reported, but little is know about its frequency because of the difficulty of making a definite diagnosis.
Diagnosis
Microbiological analysis in blood. Direct pathogen detection by culture is feasible but of no diagnostic value. PCR has not been generally adopted, as its sensitivity in identifying borrelial genomes in the CSF of patients with neuroborreliosis is only about 30% (Table 5.18). Microbiological diagnosis is still based on indirect detection of the pathogen, for which various methods have been developed (indirect hemagglutination test, complement fixation test, indirect immunofluorescence test, ELISA, and immunoblot). Generally accepted are second-generation ELISA systems (Table 5.19) and the recombinant immunoblot (Fig. 4.12). Often, ELISA is used for screening and when it is positive the result is confirmed by recombinant immunoblot. This approach is necessary only because the antigen coatings and ELISA systems of various manufacturers result in insufficient sensitivity and variable specificity overall. This is different for CSF analysis (see below).
Pitfalls in the Diagnosis of Borreliosis
• Antigens: Commercially available test systems are not standardized and use different antigen combinations. Antigen extracts from various Borrelia strains containing the highly crossreacting flagellin antigen p41 are used alongside mixtures of purely recombinant antigens without p41 and VlsE antigens, either alone or in combination with extracts. Hence, the coatings range from the p41 antigen to a combination of recombinant antigens from all three genospecies that are pathogenic to humans. In more recent test systems, the problem of the high cross-antigenicity of Borrelia seems to have been solved by preabsorption or by omitting the crossreacting antigens.
• Antibody persistence: As with many other infections (see, e. g., Fig. 19.5), antibodies may persist over decades and therefore are not conclusive evidence of acute infection. Endemic infection in the general population is 5–10%, in risk groups (e. g., forest workers) even as high as 50%. So far it has not been possible to establish test systems that allow the distinction between a “serological scar” and fresh infection. Neither the detection of IgM antibodies in the blood nor the antibody concentration are helpful in making this distinction. The importance of serodiagnosis lies strictly in the detection of a specific immune response, and the result must be considered in the entire clinical context. Especially in disease stages 1 (20–80%) and 2 (50–90%), antibody detection may be negative due to a delayed immune response. Antibodies are detected 4–6 weeks after infection at the earliest, and sometimes not until after 12 weeks. Among patients with MS, intrathecal synthesis of Borrelia-specific antibodies has been reported in up to 25% of cases as part of a polyspecific reaction.
CSF analysis. In patients with neuroborreliosis, the CSF usually shows inflammatory changes and a typical pattern of dominant IgM class reaction (Fig. 19.3). In cases of Borrelia induced facial paralysis, this reaction may be slightly weaker (Fig. 5.21
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree