Fig. 6.1
Immunostaining for esVEGFR-2 in tissues of human, 8–10 weeks old, embryos and fetuses. (a) adrenal gland (ad), liver (li) and diaphragm (di). (b) liver. (c) intercostal muscles (im), ribs (ri), note strong immunoreactivity of tendineous parts of the intercostal muscle. (d) sympathetic ganglion (sg)
EsVEGFR-2 in Neuroblastoma
Neuroblastoma is an embryonic tumor originating from neural crest cells, which belong to the sympathetic neuroblast lineage. In normal development, these cells migrate from the neural crest and form the sympathetic nervous system. Therefore neuroblastomas usually develop within or nearby sympathetic ganglia, for example along the sympathetic trunk or the adrenal medulla. Both of the latter tissues display esVEGFR-2 positivity in human embryos and fetuses (Fig. 6.1a, d). It remains unclear whether the malignantly transformed cells follow their normal pathways in the embryo or whether the malignant transformation occurs when the cells have reached their final destination.
VEGF-C-induced lymphangiogenesis and the existence of lymphatic vessels in primary neuroblastoma specimens and experimental tumors has been shown some time ago (Lagodny et al. 2007). The international neuroblastoma staging system (INSS) refers to the lymphnode status as one of the main criteria for the clinical evaluation of tumor progression and prognosis. Albeit this system has currently been revised and transferred into the International Neuroblastoma Risk Group Staging System (INRGSS), which covers more clinical imaging data, the impact of the lymphnode status is still undoubted (Monclair et al. 2009).
Therefore, we decided to investigate the expression of esVEGFR-2 in primary neuroblastoma and the clinical relevance of the expression pattern (Becker et al. 2010). Additionally, neuroblastoma cell-lines were screened for VEGFs and VEGF-receptors, including esVEGFR-2. All tested molecules were expressed in the cell-lines, but as expected for neuroblastoma, a wide heterogeneity of the expression levels could be observed. When we used the same primer sets on untreated (which means without adjuvant chemotherapy before surgery) primary tumor samples, a slight increase of VEGF-A transcripts in stage 4 s tumors and a lower expression of VEGF-D transcripts in tumors of stages 3, 4 and 4 s could be detected. Other VEGFs did not show remarkable differences between clinical stages, except for esVEGFR-2, which was expressed significantly lower in tumor stages 3, 4 and 4 s, whereas membrane-bound VEGFR-2 (mbVEGFR-2) did not show any alterations (Becker et al. 2010).
The most significant marker for neuroblastoma progression is the amplification of the transcription factor MYCN, which strongly correlates with unfavorable outcome (Westermann and Schwab 2002). MYCN amplification can be found in 20–25 % of neuroblastomas and amplification levels may reach up to several hundred copies. This leads to a massive over-expression of MYCN at transcript and protein level. Neuroblastomas with high MYCN expression are aggressive, fast growing, well vascularized and frequently metastasizing to other organs and lymphnodes. In our studies, we could not find differences between MYCN-amplified and non-amplified primary stage 4 neuroblastoma specimens for VEGF-C, VEGFR-2 and VEGFR-1 at mRNA level. However, VEGF-A and VEGF-D transcripts are moderately increased in the MYCN-amplified samples and the inhibitory soluble receptors sVEGFR-1 and esVEGFR-2 are clearly, yet not statistically significantly, down-regulated. Of note, in 26 neuroblastoma cell-lines, mainly derived from primary tumors or metastases, there were no differences between MYCN-amplified and non-amplified specimens detectable, suggesting that in vitro data and cell-line experiments may not adequately reflect tumor behavior in this aspect (Becker et al. 2010).
However, in vitro experiments using WAC2, which are MYCN-transfected and over-expressing cells derived from the neuroblastoma cell line SH-EP, confirmed the correlation between MYCN expression and down-regulation of esVEGFR-2 (Becker et al. 2010). Other Groups reported earlier that MYCN amplified neuroblastomas up-regulate VEGF-C and that VEGF-A is down-regulated after siRNA-mediated knock-down of MYCN (Eggert et al. 2000; Kang et al. 2008). These data suggest that up-regulation of pro-angiogenic factors is one feature that facilitates neuroblastoma progression, but on the other hand, down-regulation of inhibitors of angiogenesis namely esVEGFR-2 and sVEGFR-1 may also severely disrupt the balance of pro- and anti-angiogenic factors and may strongly contribute to neuroblastoma progression.
EsVEGFR-2 Expression Correlates with Differentiation in Neuroblastoma
Typically for embryonic tumors, most neuroblastomas are diagnosed in the first 2 years of life or, with the help of modern imaging techniques even before birth. The median age of neuroblastoma patients is about 24 months. Interestingly many neuroblastomas of younger patients respond better to chemotherapy or even differentiate spontaneously to benign ganglioneuroma or undergo complete regression. Therefore statistically, younger age strongly correlates with favorable outcome. In the neuroblastoma staging system, attention has been given to this fact with the introduction of the stage 4 s, which encloses children with progressed neuroblastoma including some degree of metastases to the skin and liver, but with an age not exceeding 12 months. Despite metastases formation these patients share an excellent prognosis and can often be cured. In older children with high risk neuroblastoma, retinoic acid derivatives have successfully been used to increase survival when applied together with or after conventional chemo-therapy (Masetti et al. 2012). The differentiating effect of retinoic acid on neuroblasoma cells in vitro has been shown in 1982 by Sidell and was refined in the years since (Sidell 1982; Reynolds et al. 1994).
We sought to identify whether esVEGFR-2 expression is correlated with differentiation in neuroblastoma. By immunohistology using an antibody raised against the specific C-terminal intronic peptide of esVEGFR-2, we found expression predominantly in low grade neuroblastomas (Hughes Grade I), which are stroma-rich with large tumor cells resembling differentiated neuronal cells (Fig. 6.2b). This tumor type is associated with no or only minor, regional lymph node involvement and good prognosis. In contrast, undifferentiated lesions with small round tumor cells (Hughes Grade III; WHO Grade III, IV) are often associated with distant lymph node metastases and dissemination to other organs like bone marrow or liver. This type of neuroblastoma is associated with poor prognosis and in the specimens we probed, no positive staining for esVEGFR-2 was detectable (Fig. 6.2a) (Becker et al. 2012). Especially in neuroblastoma with a histology of the differentiating phenotype, expression was detectable in the cytoplasm of maturing neurons, and in ganglioneuroblastoma and ganglioneuroma (Hughes Grade I) most of the ganglionic cells were strongly positive. This suggests that sympathetic neuroblasts might be able to inhibit tumor lymphangiogenesis by the production of esVEGFR-2.
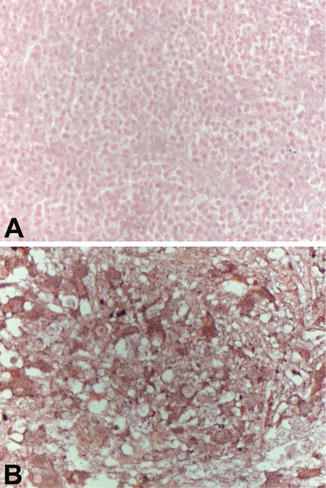

Fig. 6.2
Expression of esVEGFR-2 in human primary neuroblastoma. Paraffin sections of primary neuroblastoma specimens were subjected to immuno-histochemistry using anti-esVEGR-2 antibodies and horseraddish peroxidase-coupled secondary antibodies. (a) Neuroblastoma of the undifferentiated small round cells type shows no immunoreactivity for esVEGFR-2. (b) Neuroblastoma of the differentiating type: large neuron-like cells show up with strong immunoreactivity for esVEGFR-2 in the cytoplasm
Therapy with ATRA induces differentiation of the neuroblastoma cells and can stop malignant progression of the tumor especially in children younger than 18 months of age (Reynolds et al. 2003). Treatment of neuroblastoma cell-lines with 5–10 μM ATRA induced the maturation of the cells in vitro, which is depicted microscopically by the formation of neurite-like protrusions and by a proliferation delay or even arrest. Screening of ATRA-treated neuroblastoma cells by real-time RT-PCR revealed that esVEGFR-2 is constantly up-regulated over 12 days of treatment in SMS-KAN cells, however not all of the tested cells responded at the same degree (Becker et al. 2012).
EsVEGFR-2 in Other Cancers
So far, the effects of esVEGFR-2 have not been studied in other human cancer entities. Shibata and colleagues used a mouse model with virally inducible mamma carcinoma (Shibata et al. 2010). They treated the experimental mouse tumors with plasmid vectors containing esVEGFR-2 cDNA, introduced into the carcinoma cells by in vivo electroporation. After such treatment, tumor volumes were reduced by 30 % while survival rates increased from 60 % in controls to 90 % in esVEGFR-2-treated animals. Also, metastases to lymph nodes and the number of metastatic foci in organs like the lung were reduced by 50 %. However the number of organs (lung, kidney, adrenal and ovaries) with metastases was not altered, suggesting that the hematogenic spread of tumor cells was not affected. Remarkably, only the number of lymphatics was significantly decreased in treated tumors, while the blood vessel number was not altered. Even more interestingly, the number of lymphatics with tumor cells in the lumen decreased by about 25 % in treated tumors, which is far more than one would assume by the mere reduction of vessel number, indicating that esVEGFR-2 may also affect tumor-vessel interactions. For carcinoid cancer, a tumor derived from enterochromaffin cells of the gut, Silva et al. (2011) reported that migration in vitro is enhanced by VEGF-C binding to VEGFR-3. The authors speculate that the observed expression of esVEGFR-2 in carcinoid tumor cells may bind VEGF-C and thus slow down tumor progression (Silva et al. 2011).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








