Fig. 1
Brain sections at the bregma level show enlarged lateral ventricle area within experimental rats compared with control rats
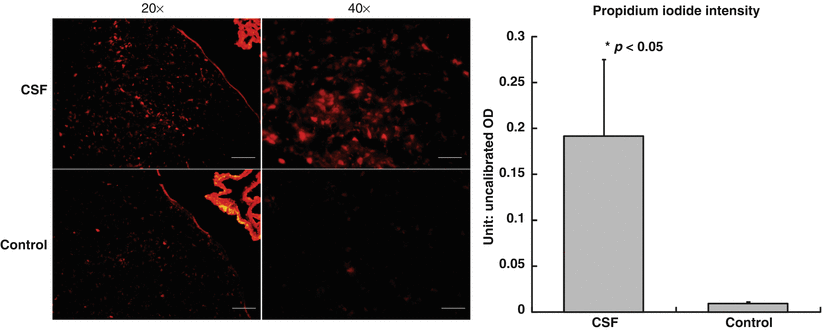
Fig. 2
Greater cellular necrotic death as evident by propidium iodide staining within the experimental group compared with the control group of rats
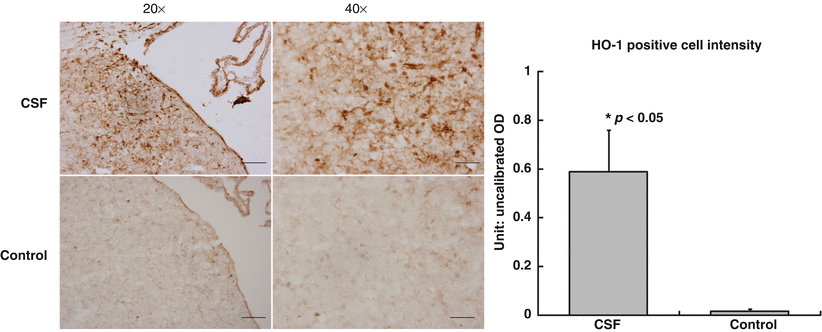
Fig. 3
Periventricular HO-1 expression, a marker of oxidative injury, was much greater in the experimental group compared with the control group of rats
Discussion
We aim to understand the role of human SAH noncellular CSF in causing hydrocephalus and periventricular cellular injury toward the goal of understanding the underlying mechanisms. We were able to show that the experimental group of rats, intraventricular injection of SAH CSF, had larger ventricles as compared with control rats, intraventricular injection of control patient CSF. Although this outcome could be related to immune or inflammatory reactions related to cross-species reaction, we controlled for this by having the control group also have intraventricular injection with human CSF. Thus, any inflammatory changes should also be present within the control rats.
Our previous studies [7, 8, 10] successfully proved that different solutions, such as iron, thrombin, and lysed red blood cells, have the possibility to induce a hydrocephalus regardless of the real concentration of these content in CSF. Shim et al. [11] analyzed the concentration of vascular endothelial growth factor (VEGF) in the CSF from patients with hydrocephalus and injected a VEGF solution with similar concentration into the lateral ventricle of rats and also succeeded in establishing a hydrocephalus model. Nonetheless, numerous biochemical components are present within the SAH CSF and, most likely, have a complex interplay in causing hydrocephalus. We aim to evaluate this mechanism by mixing inhibitors of certain pathways to understand whether SAH CSF injection will continue to cause hydrocephalus.
Propidium iodide is an intercalating and fluorescent agent, which is impermeable to membrane and excluded from viable cells. However, PI is able to penetrate necrotic cells and is incorporated into the nucleic acid [12]. The experimental group of rats had increased PI and HO-1 staining within the periventricular region, thus representing greater cellular death as well as oxidative injury in rats injected with SAH CSF.
Our study is limited by the fact that human CSF was injected within rats, thus immune reactions are to be expected and could be contributing to the changes observed. In addition, our current study represents rats that were only injected with one patient’s SAH CSF and thus injection with more patients’ SAH CSF will increase the power and reliability of the results.
Acknowledgment
This study was supported by grants NS073959, NS079157, and NS084049 from the National Institutes of Health and 973 Program-2014CB541600.
Conflict of Interest Statement
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








