Fig. 54.1
Cervical, thoracic, and lumbar musculature
Table 54.1
Muscles and ligamentous structures in the cervical, thoracic, and lumbosacral regions
Cervical | Thoracic | Lumbosacral |
|---|---|---|
Sternocleidomastoid | Trapezius | Quadratus lumborum |
Scalene | Rhomboids | Longissimus Lumborum |
Spinalis Cervicis and Capitis | Levator scapulae | Iliocostalis Lumborum |
Semispinalis Cervicis and Capitis | Longissimus Thoracis | Sacroiliac ligament |
Longus Capitis | Iliocostalis Thoracis | Gluteus medius |
Longus Colli Cervicis | Spinalis Thoracis | Gluteus maximus |
Longissimus Cervicis and Capitis | Supraspinatus | Piriformis |
Iliocostalis Cervicis | Infraspinatus |
Indications
Trigger points are the result of local contraction of a small number of muscle fibers within a muscle, as opposed to contraction of the entire muscle. The taut bands in a trigger point are due to the shortened sarcomeres in a muscle fiber. Numerous factors can contribute to the development of trigger points. Among these factors are muscle strain, postural or mechanical imbalance, overuse syndromes, muscle overload, direct trauma, and physical or psychological stress. Muscle damage is not a requisite for the development of trigger points, but biochemically, there may be associated injury to cell membranes or sarcoplasmic reticulum resulting in release of calcium-ions, and disruption of cytoskeletal proteins [1]. Presence of excess calcium-ions interferes with release of actin from myosin and hence with relaxation of muscle fibers. Sustained contraction may cause localized muscle ischemia, which in turn may result in lower pH and release of inflammatory mediators in muscle tissue. Another theory suggests that there is release of excessive acetylcholine, which can result in increased muscle fiber contractions [1].
Evidence Based Rationale
Trigger point injections are often used to treat myofascial pain. Many studies have been performed to evaluate the outcomes of trigger point injections. One study showed that lidocaine injection into myofascial trigger points improves pain and quality of life [2]. Another study found that injection of lidocaine was superior to placebo or dry needling in the short term [3]. Trigger point injections can also lead to statistically significant improvements in pain, range of motion, and depression scores with both local anesthetic injection and dry needling of trigger points [4]. A prospective randomized evaluation of treatments for myofascial trigger points argues that dry needling provides as much relief as when local anesthetic or corticosteroid are injected [5]. Therefore, local anesthetic injection, steroid injection, and dry needling have all be shown to be effective options to treat trigger points.
Technique
Trigger points are identified by palpation over the painful region. A taut band of muscle is identified. Palpation over this region produces both local and radiating pain that is concordant to the patient’s subjective report of his own pain symptoms. Though some may use EMG or nerve stimulation to confirm intramuscular needle placement and ultrasound to visualize affected muscles and taut bands, the procedure is most commonly performed without the need for additional equipment [6]. Placement of the needle into the trigger point may produce a visible and palpable muscle “twitch response.” Once the needle is in place, medication can be injected or dry needling can be performed. Some advocate directing the needle in multiple planes within a trigger point area in order to maximize the mechanical breakdown of the taut band. Some advocate the use of other injectates in trigger point injections such as botulinum toxin or prolotherapy. Platelet-Rich Plasma (PRP) is another option for the injection of painful soft tissue, but injection over ligamentous, tendinous and articular injuries are preferable to injection over muscle.
Botulinum toxin inhibits the ability of muscle to contract by interfering with presynaptic release of acetylcholine. Studies have not demonstrated superiority of botulinum toxin over other forms of injectate [3, 7]. One study suggested that the degree and duration of pain relief, improved function, and patient satisfaction was comparable between injection of Botox and injection of local anesthetic. However, botulinum toxin injection seems to improve pain scores and quality of life when compared to dry needling [2]. In a review of five clinical trials, one trial concluded that botulinum toxin was effective in reducing pain, while four concluded that it was not [8]. Another study injected botulinum toxin directly into trigger points but did not improve cervicothoracic myofascial pain [9]. It is also important to note the risk of dysphagia and other adverse effects that are associated with the use of the botulinum toxin. While cost of botulinum toxin can be a drawback and results of various studies are mixed, botulinum toxin injection into trigger points could be considered after patients have tried other measures without efficacy. Botulinum toxin is FDA approved for the treatment of chronic migraines, upper limb spasticity, cervical dystonia and detrusor muscle herperactivity. Any other intramuscular utilization of Botulinum toxin is considered an “Off-Label” use [10, 11].
Prolotherapy is a treatment for musculoskeletal pain that involves the injection of a substance, commonly dextrose, phenol, and or sodium morrhuate solution, to initiate an inflammatory reaction in soft tissue. The theory behind this treatment involves the release of inflammatory mediators that eventually recruit fibroblasts and other local growth factors to the injected areas. The influx of growth factors in theory helps rebuild stronger, healthier soft tissue, though this is yet to be fully established [12]. Patients should expect potential increased pain, swelling, and the redness for several days following the injection. These reactions signify the inflammatory and proliferative processes that the injection was intended to trigger. Risk of the treatment includes infection and increased pain with greater than intended inflammation. However, the risk is generally low, and prolotherapy can be considered for treatment of painful musculoskeletal conditions when other treatments have failed.
The PRP injection is a form of prolotherapy in that it also involves injecting a substance to promote growth factors [13]. The injectate making up PRP is an autologous blood product obtained through a centrifugal process that isolates a high concentration of platelets and growth factors from whole blood [14]. Many manufacturers market devices that utilize the centrifugal process to isolate PRP products, which are in turn injected into soft tissues or joints. This process allows clinicians to inject supra-physiologic concentrations of growth factors to stimulate and accelerate tissue proliferation. Its use in musculoskeletal medicine has increased as evidence emerges that it may augment healing in muscles, tendons, and ligaments [15].
While overall evidence for prolotherapy and PRP are conflicting, the most promising evidence for prolotherapy and PRP has been with the treatment of chronic low back pain and ligamentous injuries and tendinopathy. A Cochrane Database review concluded that prolotherapy may help improve chronic low-back pain and disability when used adjunctively with other treatments. Prolotherapy can also effectively treat refractory tendinopathies, such as in studies on lateral epicondylosis and Achilles tendinopathy. A single-blinded randomized clinical trial concluded more rapid symptom improvement in patients receiving prolotherapy and eccentric exercises for Achilles tendinopathy than with eccentric exercises alone [16]. A systematic review of injection therapy including prolotherapy and PRP for lateral epicondylitis concluded that there is strong pilot-level evidence supporting the use of prolotherapy and PRP [17]. A retrospective study evaluating PRP at various sites of chronic tendinopathy noted an overall 75 % decrease in pain, and 85 % patient satisfaction rate [18]. There is emerging evidence supporting prolotherapy and PRP in cases of osteoarthritis [17, 19]. A randomized-controlled trial showed that PRP was better than placebo in treating symptoms in patients with early knee osteoarthritis, but also noted that the benefits began to deteriorate after 6 months [20]. Preliminary results of another randomized-controlled trial suggest that PRP improves clinical symptoms of moderate osteoarthritis at 1 year follow-up. The same study did not find statistically significant differences between the benefits of PRP and hyaluronic acid, though there was a trend towards PRP in patients with lower grade osteoarthritis [21]. A separate prospective comparative study concluded that PRP showed higher and longer-lasting improvements in pain, symptoms, and recovery of articular function [22]. There are also some studies on small animals using PRP to treat intervertebral disc degeneration, with a trend towards a protective effect on damaged discs especially when utilized earlier in the disease process [13–27]. However, existing studies are limited by small sample size and design. In addition, high-level clinical trials that prove the efficacy of PRP remains lacking, especially when comparing PRP to other forms of injections and when considering the most appropriate volume, preparation, timing, and number of injections. Further research on the topic will help develop protocols and indications.
Facet Joint Injections/Medial Branch Blocks and Radiofrequency Ablation
Facet joint injections, medial branch blocks, and medial branch radiofrequency nerve ablation are strategies employed to treat pain from the zygapophysial (facet) joints. Facet joints are true synovial joints composed of the superior and inferior articular processes of adjacent vertebrae surrounded by a fibrous capsule.
Indications
Facetogenic pain is commonly due to traumatic or degenerative causes [28]. Post-traumatic facet pain can be caused by high velocity flexion from rapid deceleration injuries or hyperextension resulting in capsular stretch or joint compression [29, 30]. Facet pain from degenerative spondylosis/spondylolisthesis can lead to gradual onset of pain localized to a portion of the axial spine (i.e., high cervical, and lower lumbar) [31]. Isolated facetogenic pain is associated with myofascial radiation patterns. Facetogenic pain can be associated with radicular patterns of pain when facet hypertrophy leads impingement of exiting or traversing nerve roots.
Evidence Based Rationale
Clinical diagnosis can help guide physicians, but findings are generally nonspecific for facetogenic pain. The most specific way to diagnose facet pain is by utilizing medial branch blocks or intra-articular facet joint injections [31]. Local anesthetic is injected to provide short term pain relief for diagnostic purposes, which can assist in determining candidates for radiofrequency denervation. Medial branch blocks may provide longer lasting pain relief in a small percentage of patients [32]. There is moderate evidence to support benefits of medial branch blocks. Randomized, placebo-controlled and double-blinded studies have shown significant pain relief with radiofrequency nerve ablation, indicating strong evidence for its benefits [33]. Intra-articular steroid facet joint injections may be both diagnostic and have therapeutic benefits [34]. Available evidence from randomized, controlled trials and observational studies for benefits of intra-articular facet joint injections is mixed and rated moderate to limited.
Techniques
Medial Branch Blocks
The medial branch nerve is a terminal division of the dorsal rami of a spinal nerve. It provides sensation from the facet joints and segmental motor innervation to the multifidi muscles. In the cervical spine, the medial branch courses around the waist of the articular pillars. In the thoracic spine, the medial branches course over the superior aspect of the transverse process. In the lumbar spine, the medial branch is at the junction between the superior articular process and the transverse process [35]. Each facet joint is innervated by the medial branches from the same vertebral level and the level above it. Therefore, when attempting to block pain transmission from a facet joint, medial branches must be blocked at both of these levels. One or two sets of medial branch blocks resulting in 50–75 % or greater pain reduction is necessary prior to proceeding with radiofrequency ablation (RFA). RFA for facet generated pain has been shown to be an effective means of managing axial back and neck pain [28–35].
Cervical medial branch blocks can be performed with the patient prone or side lying. The prone positioning is aided by having the patients head turned to the contralateral side of treatment which removes the jaw from the fluoroscopic beam allowing clear visualization of the lateral aspect of the cervical spine. The fluoroscopic beam is then oriented with slight caudal tilt in line with the facet joint plane. The needle is initially placed at the lateral aspect of the waist of the articular pillar. Then the fluoroscope is placed in lateral position and the needles are advanced along the lateral border of the pillar to the mid-coronal plane at each desired level. In a lateral decubitus position, a lateral view of the cervical spine is used to target the mid coronal plane of the cervical articular pillar. Care should be taken with either approach not to advance the needle too far anteriorly past the anterior portion of the articular pillar (Figs. 54.2 and 54.3).
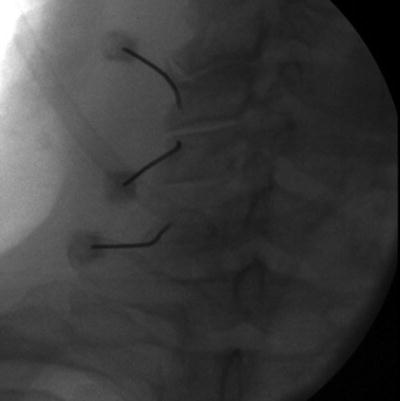
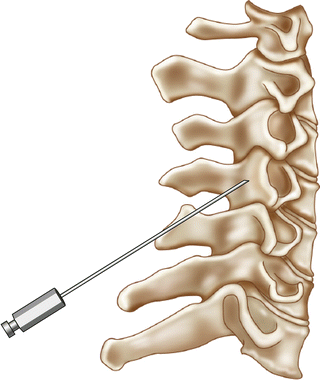

Fig. 54.2
AP fluoroscopic view of left cervical medial branch block

Fig. 54.3
Lateral illustration of cervical medial branch block
Lumbar medial branch blocks are performed with the patient in a prone position. In an AP or slightly oblique fluoroscopic approach, the spinal needles are directed towards the superolateral aspect of the pedicle at the junction of the superior articular process and transverse processes (Figs. 54.4 and 54.5).
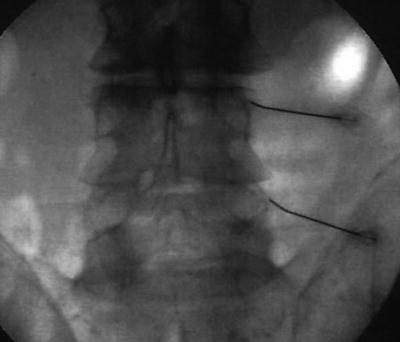
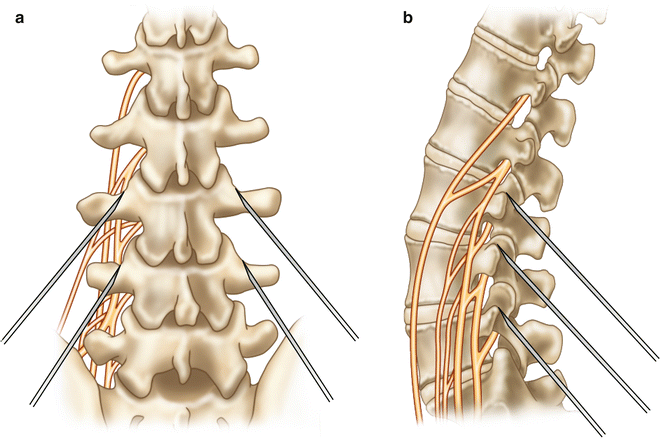

Fig. 54.4
AP fluoroscopic view of right lumbar medial branch block

Fig. 54.5
(a) AP and (b) lateral medical illustration of bilateral lumbar medial branch block
Thoracic medial branch blocks are performed with the patient in a prone position. The needles are directed towards the superior aspect of the transverse process. For levels T5–T8, the course of the medial branches run slightly superior and dorsal to the typical target point at other thoracic levels and adjustment of the needle target should be made to accommodate this variation [36].
RFA of the medial branches is performed using insulated needles place in a similar location to the medial branch block. Sensory and/or motor stimulation along with fluoroscopic confirmation of location are used to accurately place probes in proximity to the medial branch. Local anesthetic is used and then the RFA is performed. Temperature and duration settings of RFA vary but several studies have used settings around 80 °C for a duration of 60–90 s to perform the RFA [37] (Fig. 54.6).
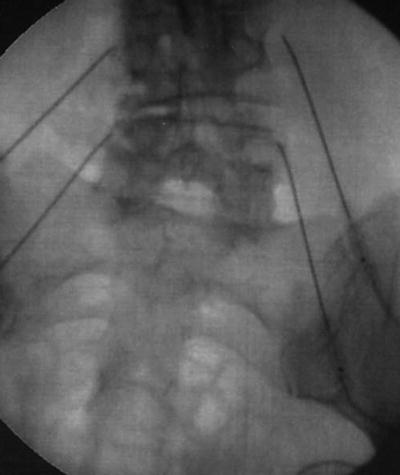

Fig. 54.6
AP fluoroscopic view of bilateral lumbar medial branch radiofrequency ablation
Facet Joint Intra-articular Injection
The target of the facet joint injection is within the joint capsule between the superior and inferior articular process. Cervical facet injections are performed with the patient lying prone. A 25–35° of caudal tilt of the fluoroscope lines up visualizes the joint space. The spinal needle is advanced towards this space. Slight resistance is felt once the needle contacts the joint capsule. Lateral fluoroscopic views can be used to assess depth. Once needle is advanced through the capsule into the joint space, medication is injected. The cervical facet joint holds approximately 0.5–1 mL. Injecting too much fluid may cause distention and injury to the capsule. For C1–C2 facet joint injections, opening the patient’s mouth will provide a clearer image. Injection at this level can be particularly risky as the vertebral artery lies very close to the lateral portion of the joint and the C2 nerve root lies over the medial half of the joint space. Therefore, it is important to keep the needle directed over the lateral third of the joint space. The use of contrast and non-particulate steroid is recommended for injection at this level given the higher risk of intravascular injection and arterial embolism from particulate steroids, which could lead to stroke. Direct injury to the spinal cord is another complication if the needle position is too deep and too medial.
To perform thoracic facet joint injections, the appropriate level is identified, and the fluoroscope is placed in a far (50–60°) caudal tilt. Thoracic facet joints cannot be fully visualized even at this extreme fluoroscope position and the steep angle of the joint line allows access to only the most inferior and posterior portion of the facet joint. However, the inferior articular process can be visualized and the needle is directed at a shallow angle towards its lower portion. Lateral fluoroscopic view can be used but anatomy at here makes assessing depth difficult. Once needle is in place, medication is injected into the joint, the thoracic facet joint holds up to 1 mL of fluid.
The patient is positioned prone and the fluoroscope is rotated obliquely to one side approximately 20–30° to perform the lumbar facet joint injection. The intra-articular joint space between the superior and inferior articular processes is visualized. The spinal needle is advanced towards this space. Slight resistance is felt once the needle passes through the joint capsule. Contrast may be used to confirm intra-capsular location. Once needle is in place medication is injected into the joint, the lumbar facet joint holds approximately 1–1.5 mL of fluid.
Facet joint injections are relatively safe with low risk of bleeding and infection. The most common complication is the risk of increased pain after the injection. Injection of too much volume can stretch the capsule and cause pain. Needle contact with the articular surface, may cause pain. Cervical injections are inherently more risky due to the denser arrangement of nerves and arteries. Accidental injection into the vertebral arteries can be particularly dangerous; arterial embolism from particulate steroids could lead to stroke and neurologic compromise. Another complication is injury to the spinal cord if the needle placed too deeply and medially [33, 34].
Epidural Injections
The epidural space is the area surrounding the dural sac in the spinal canal and extending to the brain. In the spinal canal, the epidural space is bordered anteriorly by the posterior longitudinal ligament, laterally by the pedicles, neuroforamina and nerve roots, and posteriorly by the lamina and ligamentum flavum. Epidural fat and blood vessels are present in certain areas within the epidural space. Spinal nerve roots exit the vertebral column through the vertebral neuroforamen, making this an area of interest for targeted injections. The cervical neuroforamen faces obliquely anterior and lateral, whereas the thoracic and lumbar neuroforamen faces obliquely posterior and lateral. Neuroforamen are bordered superiorly and inferiorly by the pedicles of the adjacent vertebra, anteriorly by vertebral bodies and intervertebral discs, and posteriorly by the facet joints.
Indications
The primary indication for epidural steroid injections is radicular pain. Radicular pain occurs when there is injury or inflammation of the spinal nerve root due to mechanical or chemical irritation. This may be due to a disc herniation impinging upon the nerve, or other causes of central spinal or neuroforaminal stenosis. It is classically described as achiness in the proximal portions of a limb with associated shooting pain, numbness and paresthesias along a dermatomal pattern. Pain radiating along dermatomes to the upper and lower extremities suggest cervical and lumbosacral radiculopathy respectively, while dermatomal pattern of pain along the anterior thoracic wall suggests thoracic radiculopathy (Fig. 54.7). Clinical history of patient’s pain should be correlated with physical exam (muscle weakness/atrophy, diminished reflexes, sensory changes, straight leg raise test), available imaging, and electrodiagnostic studies. Patients with primarily axial pain or whose limb pain is referred from bony, facetogenic, or myofascial sources typically do not respond appropriately to epidural injections. Epidural steroid injections are accepted as a standard treatment for radiculitis and neurogenic claudication [38]. Epidural steroid that are commonly used for epidural use do not have an FDA labeling or approval for epidural use [39].


Fig. 54.7
Dermatome chart
Evidence Based Rationale
Treatment of cervical, thoracic or lumbar radiculitis often includes the use of corticosteroids. In the acute phase of radicular symptoms, treatment options include oral corticosteroids, often a 6-day tapering dose of methylprednisolone. Studies have shown this treatment option can speed recovery from acute radiculitis [40, 41]. Epidural corticosteroid injections allow placement of medication directly at the location where its action is most desired which reduces systemic absorption and effects when compared with oral corticosteroid intake. Furthermore, targeted injection near the spinal nerve roots has diagnostic and surgical prognostic benefits [42–44]. Transforaminal epidural injections or selective nerve root blocks are of value in the preoperative evaluation of patients with clinical findings of root irritation but negative imaging studies [45]. They are also of value in helping to diagnose the source of radicular pain when imaging studies suggest possible compression of multiple nerve roots [46–56]. Some have also suggested that epidural injections could be used as a surgery-sparing or delaying intervention [57].
Comprehensive review of existing studies found that epidural steroid injections have short and sometimes long term benefits [57–60]. Patients who are most likely to have a beneficial response are those who demonstrate acute/subacute symptoms in a dermatomal distribution, with corresponding findings on imaging, clinical exam, and electrodiagnostics. Electrodiagnostic evidence suggestive of acute radiculopathy includes positive sharp waves, fibrillation potentials, and decreased recruitment in muscles innervated by the affected nerve root. Electrodiagnostic evidence suggestive of chronic radiculopathy includes increased motor unit action potential amplitude, duration, and phasicity. A comparison of the three different routes of injecting epidural steroids demonstrated that all three routes of administration were clinically effective, but caudal and transforaminal routes were more likely to obtain longer relief. This study also showed that the most significant improvement was noted with transforaminal injections when compared to fluoroscopically guided caudal, and blind interlaminar epidurals [61]. According to American Society of Interventional Pain Physicians (ASIPP) guidelines, these procedures should be repeated only as a medical necessity and limited to a maximum of six times per year. Injections should be spaced at least 2 months apart. ASIPP guidelines describe numerous studies that have been performed throughout the years on efficacy and outcomes of caudal, interlaminar, and transforaminal steroid injections. Multiple systematic reviews, randomized trials, and observational studies show strong evidence for caudal epidural steroid injections [33]. There is strong to moderate evidence supporting transforaminal epidural steroid injections [33]. In terms of quality of evidence from controlled studies, the authors conclude that outcomes are predominantly negative for lumbar interlaminar and positive for cervical interlaminar epidural injections. Overall, evidence for interlaminar injections is considered moderate to limited. However, multiple observational studies have shown positive results with all forms of epidural injections [33].
Steroids used for epidural injection include methylprednisolone (40–80 mg), triamcinolone (40–80 mg), betamethasone (6–12 mg), and dexamethasone (4–12 mg). Of these, all except dexamethasone are particulate steroids. There has been no clear evidence-based demonstration of superiority of one steroid over another. There have been some arguments suggesting particulate steroids may be marginally more efficacious than non-particulate steroids [62]. However, there have been numerous reports of CNS complications such as stroke and paralysis that were felt to be related to vascular embolism of particulate steroids. In light of these reports, some considerations have been given to non-particulate steroids being somewhat safer than using particulate steroids [63]. It is desirable to use the lowest steroid dose possible because of adverse effects of cumulative steroid administration such as osteoporosis and adrenal insufficiency among others. Suggested dosing is 3 mg/kg/year or 210 mg of methylprednisolone steroid equivalents per year limitation in an average person. A lifetime total dose of 420 mg of methylprednisolone equivalents has also been advocated [33]. Studies that suggest lower doses of steroids can achieve comparable results to higher doses with less side effects [64–66].
Technique
Three approaches can be used to inject medications into the epidural space: caudal, transforaminal and interlaminar. Transforaminal and interlaminar approaches can be used for cervical, thoracic, and lumbosacral epidural injections whereas the caudal approach is used for lumbosacral radiculopathy. A catheter can be utilized to provide a more precise delivery of injectate and decrease the need for large volumes of injectate.
Caudal
Fluoroscopic guidance is used to identify the sacral hiatus in a lateral view. A spinal needle is advanced in a cephalad direction into the sacral hiatus. A slight release can be appreciated as the needle passes through the sacrococcygeal ligament and into the most caudal region of the epidural space. Contrast solution is then injected with or without a catheter, to confirm epidural spread then followed with injectate (steroid and local anesthetic). The risk of dural puncture increases if the needle is inserted rostral to the level of S2, which is the level where the thecal sac often terminates [67]. Incidence of cauda equinea syndrome can be around 2.7 per 100,000 epidural blocks [33].
Interlaminar
Interlaminar epidural injections are performed with the patient in the prone position with slight flexion of the spine in the target area. The advantage of the interlaminar approach is that the injectate is placed in the general area of desired treatment and may also spread in the dorsal epidural space to affect multiple levels. Thus, it may more effectively target symptoms from multilevel spinal disease [68] (Fig. 54.8).
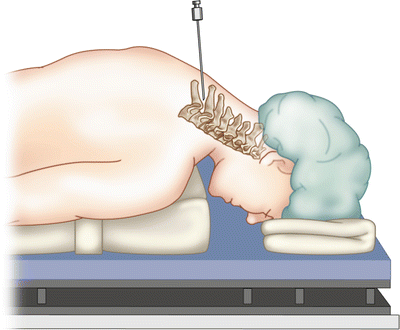

Fig. 54.8
Positioning for cervical interlaminal epidural injection
A paramedian approach is recommended. The spinal needle is advanced to the junction of the spinous process and the inferior lamina of the intralaminar space under fluoroscopic guidance. Contact with the lamina validates depth of the needle tip. The needle is then marched off the superior edge of the lamina (Fig. 54.9). The needle can then be advanced with or without lateral fluoroscopic guidance using either pneumatic or hydraulic loss of resistance through the ligamentum flavum into the epidural space. Many practitioners prefer Hydraulic Loss of resistance using a glass syringe and saline [69]. Aspiration is performed prior to injection of contrast to check for blood or CSF. The potential size of the dorsal epidural space is directly related to the volume of the spinal canal at the targeted level [70, 71].
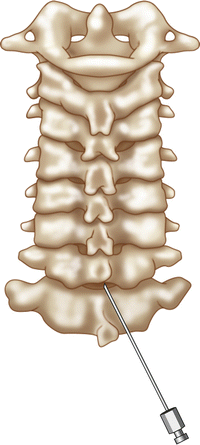

Fig. 54.9
Paramedian approach for cervical interlaminar epidural injection
Cervical interlaminar injections are commonly performed at or above the level of the suspected pathology associated with pain. The space between thoracic vertebrae is better visualized by more pronounced caudal angulation of the fluoroscope. This is due to the “shingling” of the thoracic lamina. Thoracic interlaminar space is largest at the most cephalad and caudal segments of the thoracic spine. A catheter can be used to access the mid-thoracic segments when necessary. The authors recommend access at the T1–T2 or T2–T3 interlaminar space and utilization of a soft tip catheter for cervical epidural injections. The dorsal epidural space is larger in the thoracic spine and flexion of the cervical spine reduces lordosis which increases interlaminar space and improves catheter navigation when advanced up the cervical spine (Fig. 54.10).
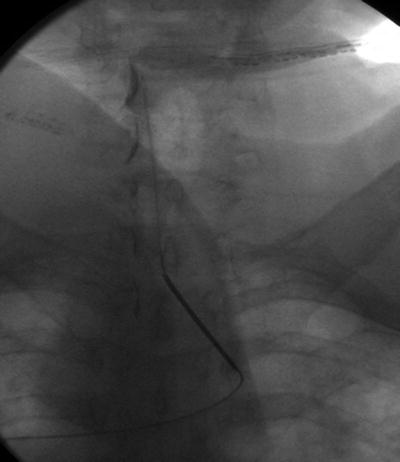

Fig. 54.10
AP fluoroscopic view of cervical interlaminar epidural steroid injection with entry point at the T1–T2 interlaminar space
Transforaminal
Previous publications claim the transforaminal epidural approach leads to greater benefit than interlaminar or caudal [57, 72, 73]. The retroneural thoracic and lumbar transforaminal approach requires prone positioning with a lateral to medial needle approach with the fluoroscopic beam at a 45° oblique angle on the ipsilateral side of the targeted side. The needle is advanced toward the 6-o’clock position of the pedicle at the junction of the transverse process and the pars anteroposterior (AP) and lateral views are then used to ensure the needle tip is in the “safe triangle” at the superior dorsal quadrant of neural foramen (Figs. 54.11, 54.12, 54.13, and 54.14). The S1 neuroforamen can also be visualized and targeted in this view, but is more traditionally targeted in the AP view [74]. Cervical transforaminal epidural injections are not advised for unskilled proceduralist. Since 2002, there have been numerous case reports that describe paralysis, stroke, and death [63, 75].
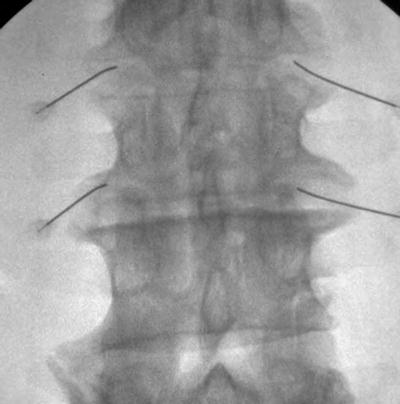
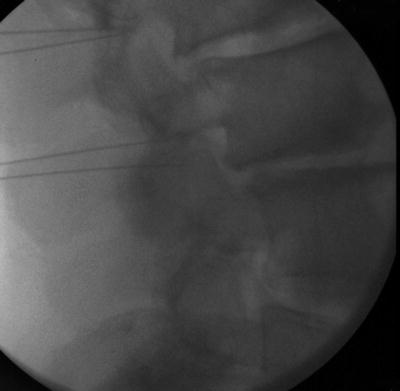
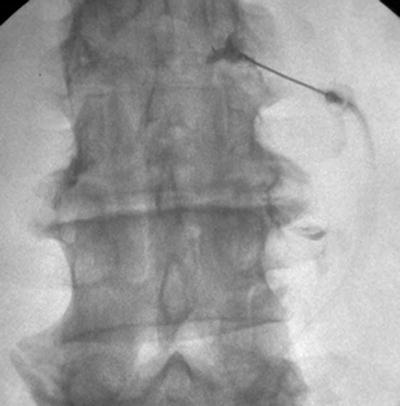
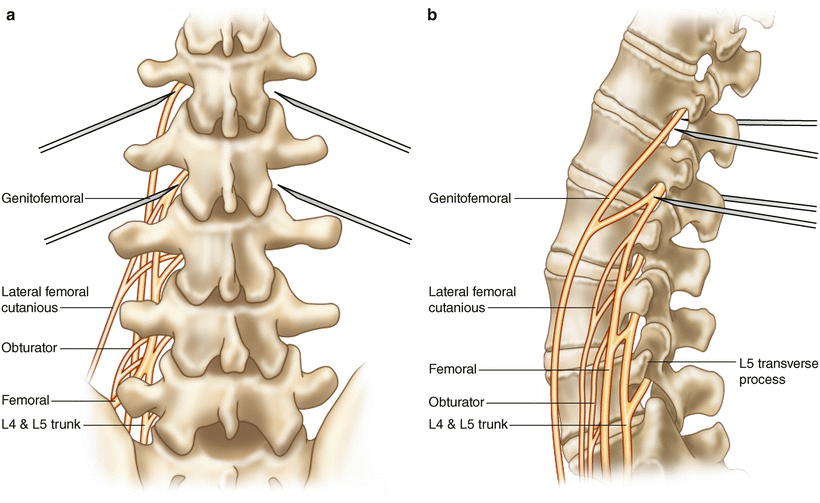

Fig. 54.11
AP fluoroscopic view of lumbar transforaminal epidural steroid injection with needles in place

Fig. 54.12
Lateral fluoroscopic view of lumbar transforaminal epidural steroid injection with needles in place

Fig. 54.13
AP fluoroscopic view of lumbar transforaminal epidural steroid injection after administering contrast and medications

Fig. 54.14
(a) AP and (b) Lateral medical illustration of lumbar transforaminal epidural steroid injection
Complications
Complication rate for epidural steroid injections are reported to be low. A study by Mcgrath et al. reported an overall complication rate of 2.4 % with the most common complications being increased pain (1.1 %), pain at injection site (0.33 %), and persistent numbness (0.14 %) [76]. Another study reports infections in 1–2 % of all spinal injections with severe infections (meningitis, osteomyelitis, epidural abscess) even rarer with an incidence of 0.1–0.01 % Epidural hematoma occurs in approximately less than 1 in 150,000 epidural injections. Intravascular injection with transforaminal approach is at 11.2 % versus 1.9 % for interlaminar approach [77].
Should one encounter larger amounts of clear return upon entry of the spinal canal, a litmus test can be performed to verify if CSF is present. Most dural punctures heal without requiring intervention, but patients with persistent dural leaks and headache may be treated with hydration, rest in supine position, analgesics, and/or autologous blood patch [78]. Direct needle injury to the spinal cord is also possible when performing injections at levels L2 and above. Other complications include epidural infection and hematoma, which in turn may compress the spinal cord, nerve roots, or cause a cauda equina syndrome. Vascular injection of steroids has been related to devastating neurologic injury when arterial in nature. Left sided injections between T8 and L1 need to be specifically mentioned because the artery of Adamkiewicz, the largest spinal segmental artery, lies at these levels in 60–80 % of patients [79, 80]. Altered anatomy post surgical due to neovascularization and scar can increase the risk of neural or vascular injection [81].
Sacroiliac Joint injections
The SI joint is formed by the sacrum and the ilium. The majority of this apposition is formed by a fibrocartilaginous connection. However, inferoposteriorly, there is a small true synovial joint. SI injections are performed targeting this space.
Indications
Sacroiliac (SI) joint injections are performed for patients with pain suspected of arising from the SI joints, which affects between 15 and 30 % of individuals with chronic axial low back pain [82]. Sacroiliac joint pain is associated with leg length discrepancy, older age, inflammatory arthritis, scoliosis, previous spine surgery, pregnancy, and trauma. Pain from the SI joints may be unilateral or bilateral and are typically localized to the lower back and buttock region. There may also be referred pain to the proximal posterior or lateral thigh. Patients also tend to have tenderness over the border of the sacrum and ilium and pain with provocative tests such as FABER and Gaenslen’s test.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








