Insular Lobe Epilepsy
Introduction
Among all forms of epilepsy, insular epilepsy is one of the most rarely reported. Its clinical and electrophysiological characteristics are poorly understood, providing the clinician with many challenges. For a long time, there was great controversy in the literature with some early authors suggesting that seizures arising from the insular cortex could mimic temporal lobe seizures, leading to great confusion between the two seizure types. However, this concept fell out of favour, primarily because the authors in question failed to record spontaneous epileptiform discharges from a focal onset within the insular cortex. In retrospect, the insula’s close proximity to the temporal lobe allows, through spread patterns, a considerable overlap in seizure semiology between these two forms of epilepsy. Indeed, many of the difficulties posed arise from the insular lobe’s anatomy, especially its location within the cortex and its prominent connections to other cortical structures. Correct identification and evaluation is extremely difficult and requires astute interpretation of clinical findings; many of the usual tools, such as electroencephalography (EEG) or seizure semiology used by an epileptologist fail to yield exact identifying information.
Anatomy of the insular lobe
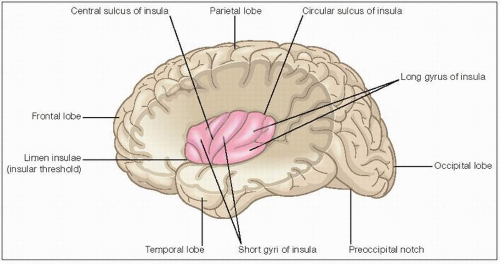
Good knowledge of insular lobe anatomy is vital for a better understanding of how the insular lobe and opercular epilepsy manifest clinically (6.1). The insula is one of the five cerebral lobes and is located deep within each hemisphere. It is buried in the depths of the lateral sulcus and superficially adjacent to the basal ganglia. It is only exposed when the overlying lips of the lateral sulcus, i.e. the frontal, parietal
and temporal opercula are pulled apart. It is separated from the opercula by a dense wall of arteries running in the lateral fissure. The insula has an approximate triangular surface and is surrounded by the circular sulcus. The ventroanterior margin, the limen insulae, is continuous with the anterior perforated substance in a ventromedial direction.
and temporal opercula are pulled apart. It is separated from the opercula by a dense wall of arteries running in the lateral fissure. The insula has an approximate triangular surface and is surrounded by the circular sulcus. The ventroanterior margin, the limen insulae, is continuous with the anterior perforated substance in a ventromedial direction.
The insula serves to integrate multimodal information from a broad number of networks. The anterior insula connects with a network of structures that are implicated in oro-alimentary behaviour among other things; connected structures include the piriform cortex, the hippocampus and the amygdala. The posterior insula has dense connections with the primary, the secondary somatosensory cortex and the parietal operculum, structures that together make up a somesthetic network. The connections and consequent functions of the insula can also be distinguished by its cytoarchitecture, divided into three fields: (1) agranular field (Ia, related to olfactory and autonomic functions); (2) dysgranular field (Id, related to gustatory functions); and (3) granular field (Ig, associated with somatosensory, auditory, and visual functions). Table 6.1 summarizes the connections of the insula and its functions.
Seizure semiology
The greatest challenge insular lobe epilepsy poses to the clinician is the similarity of its clinical features to those of temporal lobe epilepsy (TLE). This is not surprising due to its anatomical proximity to the temporal lobe and prominent connections that allow ictal spread to occur within seconds of ictal onset. The semiology is usually highly suggestive of TLE with great overlap; clonic movement and dystonic posturing have been frequently reported. Insular seizures tend to show visceral sensory, somatosensory and visceral motor phenomena, sometimes reminiscent of auras reported in TLE. Automatisms are also prominent in both TLE and insular lobe epilepsy, with lip smacking highly suggestive of propagation to the amygdala in the latter. An important difference is that in a purely insular seizure with no propagation, awareness is maintained throughout unlike a temporal lobe seizure; however, it may be lost if spread occurs early in the seizure evolution. Table 6.2 groups prominent seizure semiology that should suggest to the clinician an insular ictal onset zone.
The list in Table 6.2 is by no means complete; however, on review of case reports, these symptoms appear to be the most prominent and useful while guiding clinicians towards diagnoses, especially those regarding the throat, including excessive salivation. Later stages of the seizure might mimic a temporal lobe seizure with prominent motor phenomena, indicative of ictal spread.
Table 6.1 Connectivity and functions of insula | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Electroencephalography in insular lobe epilepsy
Again, due to the anatomical situation of the insular lobe surface EEG findings are misleading, often suggesting an ictal onset zone in the temporal lobe. Surface electrodes fail to show specific changes, usually yielding only temporal or
frontotemporal slowing. To obtain adequate sampling from the insula, depth or subdural electrodes must be used, or intraoperative electrocorticography (ECoG) must be applied. The placement of depth electrodes is not without risk. Depth electrodes must travel through the Sylvian fissure, which contains branches of the middle cerebral artery; to avoid unnecessary complications, usually three-dimensional angiography and magnetic resonance imaging (MRI) (known as stereo-EEG) or computed tomography (CT) integration is carried out to guide their placement. Subdural electrodes are easier to place via the splitting of the Sylvian fissure; however, when placed and confined in this narrow space, often a mass effect from the electrodes themselves is seen and recordings are rendered suboptimal. Placement of subdural electrodes along the mesial temporal lobe structures are also unlikely to record epileptiform discharges from the insula. ECoG is felt by many to be the easiest way to reliably record from the insula. It is performed intraoperatively after the Sylvian fissure has been split; electrodes are placed directly on the insular cortex where a focus can be identified. There are some spatial and time limitations; however, this method has shown itself to yield good electrophysiological data reliably and confirm the clinical suspicion of insular lobe epilepsy (6.2).
frontotemporal slowing. To obtain adequate sampling from the insula, depth or subdural electrodes must be used, or intraoperative electrocorticography (ECoG) must be applied. The placement of depth electrodes is not without risk. Depth electrodes must travel through the Sylvian fissure, which contains branches of the middle cerebral artery; to avoid unnecessary complications, usually three-dimensional angiography and magnetic resonance imaging (MRI) (known as stereo-EEG) or computed tomography (CT) integration is carried out to guide their placement. Subdural electrodes are easier to place via the splitting of the Sylvian fissure; however, when placed and confined in this narrow space, often a mass effect from the electrodes themselves is seen and recordings are rendered suboptimal. Placement of subdural electrodes along the mesial temporal lobe structures are also unlikely to record epileptiform discharges from the insula. ECoG is felt by many to be the easiest way to reliably record from the insula. It is performed intraoperatively after the Sylvian fissure has been split; electrodes are placed directly on the insular cortex where a focus can be identified. There are some spatial and time limitations; however, this method has shown itself to yield good electrophysiological data reliably and confirm the clinical suspicion of insular lobe epilepsy (6.2).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree