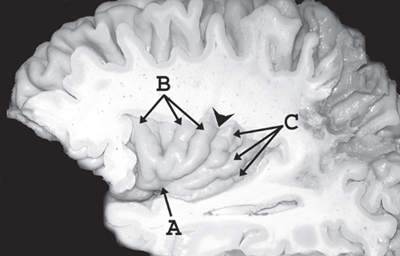
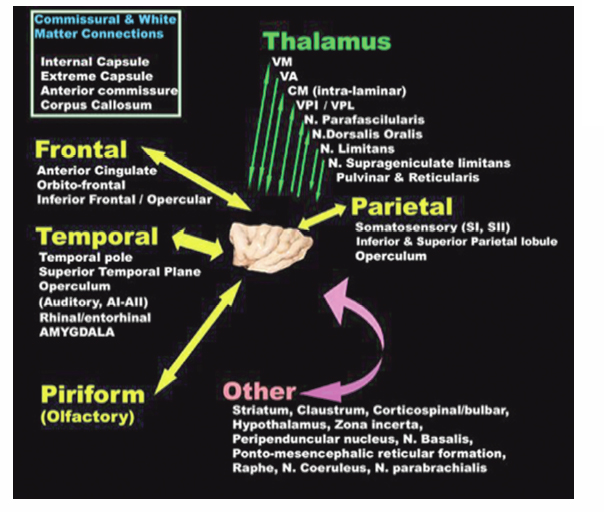
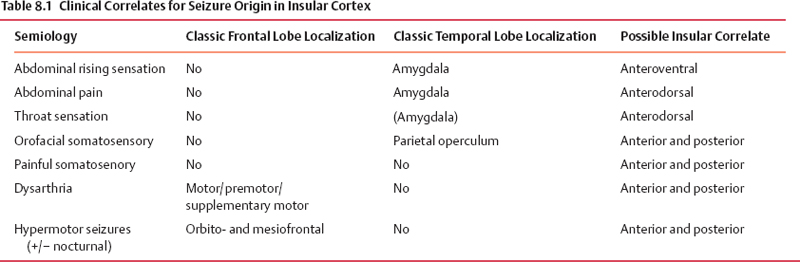
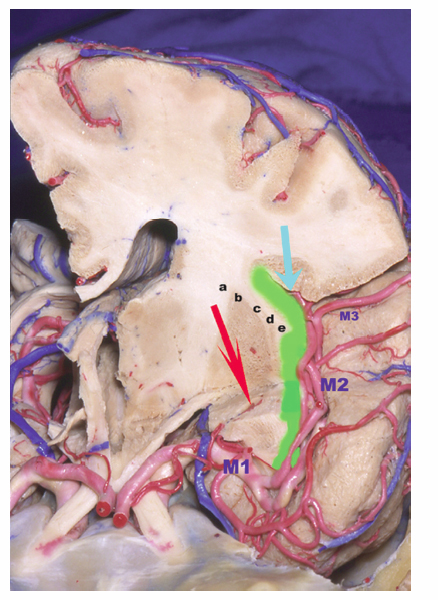
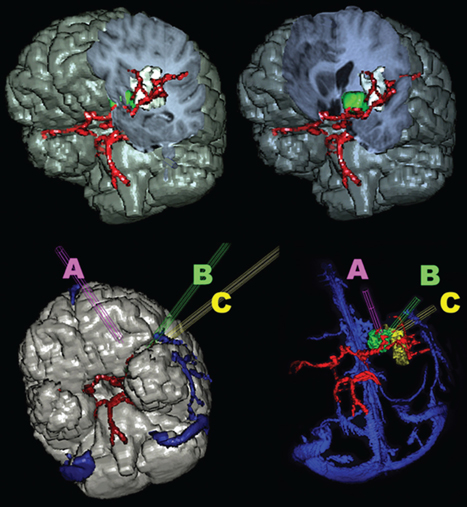
8 Insular Resection There has been a rebirth of interest in the insula of Reil from a surgical perspective over the past years. Insular cortex remains somewhat inaccessible because it is buried in the sylvian fissure (Fig. 8.1), but this area is clearly of importance in epileptogenesis and is usually considered a “paralimbic” structure.1–5 Deep to the insular cortex lies the extreme capsule, the claustrum, the external capsule, the putamen, and the pallidum (Fig. 8.2). In reports regarding “insular lesions,” it is common that these lesions involve these basal ganglia areas as well as the more superficial insular cortex.6–9 Epilepsy is a common presentation of “insular” lesions. Part of the reason for this can be presumed to be related to the paralimbic nature of insular cortex. In addition, the proximity of piriform cortex and the deep endopiriform area may also play a role in epileptogenesis. The deep endopiriform nucleus was first discovered to be a highly epileptogenic area in rats and termed area tempestas (AT).10–12 The existence of AT was subsequently supported in monkeys,13 and there are some suggestions of its existence in man.14 The presumed location in man adjacent to, or within the insular “lobe” may be a further reason for insular involvement in epileptogenesis. In “nonlesional” limbic-type seizures, there is indirect15,16 and more recently direct evidence of insular involvement as a component of the epileptogenic network.17–19 Classic awake intraoperative stimulation studies of the insula resulted in sensory and autonomic phenomenology typical of complex partial seizure semiology.20 In particular, abdominal rising sensation is thought to be associated with involvement of the amygdala and anterior insula. The stimulation of the anteroventral insular cortex has been shown to increase gastrointestinal motility along with other autonomic changes, including changes in heart rate. There has been a wealth of recent data, predominantly from France, on insular involvement in spontaneous seizures in patients.18,19 This has been derived from invasive electroencephalogram (EEG) recordings during investigation for epilepsy surgery. Most of these studies of stereo-electro-encephalography (SEEG) have been performed according to the technique described by Talairach in the 1950s, in which a stereotactic angiogram is used for frame-based placement of depth electrodes via an orthogonal approach.21 The angiogram is particularly valuable in the context of insular studies because electrodes need to safely navigate between cortical veins and the branches of the middle cerebral artery (MCA) to safely reach insular cortex. The resulting studies and publications have given us additional insight into insular function by way of stimulation studies of the insular contacts of the electrodes.17,22,23 Recording spontaneous seizures using the same technology has highlighted insular participation not only in seizure propagation, but occasionally as a locus of primary seizure onset or of early network participation. Fig. 8.1 Left cerebral hemisphere with partial frontal, parietal, and temporal lobe removals to expose the insular lobe. The insula has a fan-shaped arrangement centered on the anteroventrally located limen insula (A). The insula is morphologically divided into anterior (B) and posterior (C) parts by the central insular sulcus (arrowhead). Histologically, the limen features three-layer allocortex. Cortex takes on six-layer features as radial distance from the limen increases and the granule cell layers become more defined. With the relatively recent SEEG studies documenting the semiology of seizures with insular onset or participation, Table 8.1 establishes some criteria for clinically suspecting participation or origin of seizures in insular cortex. Unfortunately, there is considerable overlap in insular seizure semiology with not only typical temporal lobe clinical features,18 but also with some features thought to be classically implicating the frontal lobe.24–27 This may in part be due to the fact that there is a histological similarity between the anteroventral insula, the posterior orbitofrontal cortex, the piriformcortical amygdalar complex and temporopolar cortex. It may also be due to rapid seizure propagation between these areas. Traditionally, nausea, vomiting (ictus emeticus), and autonomic features have been thought to be indicative of insular but also amygdalar involvement.28 Typical “temporal lobe” semiology such as rising abdominal and thoracic sensation is in fact quite “amygdaloinsular.”29 Throat constriction or abdominal discomfort at seizure onset may be somewhat more suggestive of insular origin.19,30 More recently, nocturnal hypermotor seizures, previously thought to be typically of frontal lobe origin, can have an insular origin on SEEG.26 The search for clinically suggestive features of insular seizures is important in evaluating the necessity for invasive EEG studies with insular coverage. Fig. 8.2 Literature-based summary of insular connections. As with all epilepsy surgery, there is a disconnective component as well a resective component. This should be kept in mind with regard to seizure origin in or propagation from or to the insula. Insular cortex in man is completely hidden within the sylvian fissure, covered by frontoorbital, parietal, and temporal opercular cortices. First described by Johann Christian Reil in 1809, this cortical “island” is triangular in shape and is surrounded by a “circular sulcus” that can also be divided into anterior, superior, and inferior components bordering orbitofrontal, frontoparietal, and temporal opercula, respectively (Fig. 8.1). An oblique central insular sulcus (which parallels and is most often an extension of the central sulcus proper) separates the insula into an anterior component consisting of three (to four) short anterior gyrii and the posterior insula consisting of two long gyrii. There are also usually two short transverse gyrii at the orbitofrontal junction. Based on its connections and cytoarchitectonic characteristics, insular cortex has been classified as a paralimbic structure by Mufson and Mesulam, along the same lines as orbitofrontal, temporopolar, and cingulate cortex.1–4,31 There is immediate proximity between the piriform olfactory cortex (POC) and the limen of the insula (which constitutes the anteroinferior corner of the insula), both areas featuring three-layer allocortex. The cytoarchitectonic features of the cortex progress to six-layer neocortex with increasing distance from the POC with a transitional cortex where the granule cell layer is not yet defined (dysgranular cortex). Within the insula, this change in histology occurs in a radial fashion as distance from the limen increases. As a consequence, the anteroventral aspect of the insula, in closest proximity to the limen insula (LI) is histologically (and functionally) the most “limbic.” Figure 8.2 summarizes the neuroanatomical literature on insular connections. This is indeed the area of the insula with demonstrated autonomic connections and where awake stimulation mapping by Penfield and Faulk has elicited abdominal sensations, increases in gastrointestinal motility, and other autonomic responses.20 Changes in heart rate have also been elicited from this area, with suggestions that there may be a right insula-left insula difference in cardiac response.32,33 It is noteworthy that the insula has been implicated as having a possible role in cardiac arrhythmia and death in stroke and as a possible mechanism for sudden unexpected death in epilepsy (SUDEP).34–39 Regarding the latter, it has been postulated that epileptic activity involving the anteroventral insula may lead to cardiac arrest and SUDEP. It is of surgical importance to be aware of these autonomic effects because cardiac and blood pressure changes can occur during surgery in these areas. Within the context of hemispherectomy variants, these autonomic effects have been implicated as a possible source of instability during surgery of which anesthesiologists should be aware.40 Human awake mapping with SEEG has demonstrated gustatory responses in the more dorsal anterior insula.30 In the posterior insular cortex, sensory responses were evoked including pain responses. This is concordant with many functional imaging (positron emission tomography [PET] and functional magnetic resonance imaging [fMRI]) studies that show insular cortex activation in response to noxious stimuli.41–45 Conversely, dysarthria but no true dysphasia was elicited by SEEG stimulation.30 There is considerable literature in functional imaging and stimulation mapping that suggests some speech localization in the insular cortex in the dominant hemisphere.46,47 That any primary speech function may be localized in insular cortex is more difficult to establish because surgical resection in the dominant hemisphere affects opercular cortex, where there is definite speech localization and open stimulation studies probably activate white matter deep to insular cortex that may lead to disconnective types of speech disturbance or motor speech interference.48–51 Fig. 8.3 Coronal view of left insular anatomy. (A) Internal capsule/ corona radiate. (B) Putamen. (C) External capsule. (D) Claustrum. (E) Extreme capsule. Insular cortex is highlighted in bright green. Segments of the middle cerebral artery are labeled as M1, M2, and M3. A lateral lenticulostriate branch arising from the M1 segment is dissected out (red arrow). A distal perforating artery from the M2 branch is also illustrated (blue arrow). As previously mentioned, access to the insula is restricted by its deep location and by its close relationship to the MCA (Fig. 8.3). The insular cortex is vascularized exclusively by the MCA, and the underlying basal ganglia and internal capsule are supplied to a major degree by the M1 segment of the MCA via the lateral lenticulostriate arteries (LSAs).52–56 These usually do not extend as far laterally as the actual insular cortex, which is supplied by perforating arteries arising from the overlying M2 segments of the MCA with occasional branches off the distal M1 to the area of the limen before dividing into M2 segments in this location. There are also occasional branches from the opercular M3 segments coursing back to the insular cortex. It is particularly noteworthy that an M2 branch is consistently found in the central sulcus, which subsequently supplies the central sulcus proper, thus the pre- and postcentral gyri. It is therefore particularly important to avoid compromising this artery. Several detailed anatomical studies have reviewed the vascular anatomy and supply of the insula. From a surgical perspective, it should be noted that a motor deficit from insular surgery that does not directly lesion the internal capsule is most likely to be the result of a vascular injury at one of three levels: Based on the previously described anatomical and physiological features, a risk assessment of surgery of the insula can be attempted. In the context of surgery for epilepsy, this must be weighed against the chances of controlling seizures with the planned surgery to establish a likely risk-benefit ratio. Because there are still many unknown aspects regarding insular function, an absolute risk assessment is not possible. The current knowledge with regard to results of insular surgery to control seizures is even less clear. In the risk assessment of surgical approaches to the insula, it is useful to differentiate between lesional surgery and nonlesional surgery. Lesions that are considered “insular” in the literature most often refer to those not only involving insular cortex but also deeper underlying structures including the basal ganglia. The risks of surgical approaches to these areas is different from an approach to the more superficial insular cortex alone, in that there is additional risk of a motor deficit by direct injury to the internal capsule or by vascular injury to the same by compromise of the lateral LSAs. This compounds the motor risks of superficial insular approaches where the long perforating arteries from the M2 branches or artery of the central sulcus are at risk. Assessment of risk to speech function is not as straightforward as that for motor function. Clearly, in the dominant hemisphere, there is a speech risk in the approach itself because frontal, parietal, and temporal opercular cortices are the classic locus for primary speech function. Therefore, any resection, retraction, manipulation, or vascular compromise to opercular cortex in the dominant hemisphere carries a risk to speech function. Stimulation mapping for speech function can help lower risk to speech by helping to define opercular areas lacking obvious language function. Avoidance of resection or retraction of more eloquent opercular cortex should decrease risk to speech.7,8 As previously mentioned, evidence of primary speech localization to insular cortex itself is less convincing, although several authors who have experience with insular surgery recommend stimulation mapping of the insula itself.51,57,58 Others do not.7,59 Some interference with speech function may be more related to a disconnective effect at the level of the external capsule than an actual cortical insular lesion. This author prefers to consider awake speech mapping on a selective, case-by-case basis. Risk to higher cognitive function is the most difficult of all to discuss regarding insular surgery because the insula is the seat of multimodal integration. Anteroventrally there is evidence of autonomic processing in close conjunction with the amygdalar complex (behavior, motivation, and learning). Dorsally and posteriorly, sensory (including pain), motor, and vestibule-auditory connections exist. Functional imaging also shows insular in the context of more complex auditory processing, pitch perception, and singing.60–63 In the context of dominant hemisphere insular infarct, loss of verbal memory has been described, but these lesions are unlikely to have involved insular cortex in isolation.46,64–66 Overall, higher cognitive effects of insular surgery cannot be predicted at present. Because this chapter addresses surgery of the insula to control epilepsy (i.e., chronic intractable seizures), surgery for other indications will not be addressed. Vascular, tumor, and malformative pathologies will be addressed briefly from a practical perspective based on this author’s experience and biases. Although arteriovenous malformations (AVMs) that involve the sylvian fissure and the insula can present with chronic seizures, the treatment of these is beyond the scope of this chapter because surgery for AVMs alone is not strictly epilepsy surgery, and other modalities including interventional neuroradiology and radiosurgery play an increasingly important role in AVM management. Cavernous angiomas, on the other hand, frequently present as “lesional epilepsy” cases and can be localized to the insular cortex or insular lobe.67–72 There are no large series of insular cavernoma resections for epilepsy, but surgical feasibility of resection has been documented in case reports and series where epilepsy was not the predominant presentation. Figures 8.4 and 8.5 illustrate some of the surgical considerations involved. This patient has a cavernoma centered deep to the limen insula and has had daily seizures for 12 years. Although the cavernoma does not directly involve insular cortex, there are signal abnormalities in the overlying limen probably due to remote microhemorrhages (Fig. 8.5). This patient has not yet had surgery and has had five neurosurgical opinions. She has a high level of function. It was felt that lesion resection would probably improve her daily seizures, but she does have nine other very small cavernous angiomas in other less epileptogenic locations. Video EEG captured 16 events, eight of which showed no EEG changes, the other eight featured left frontotemporal rhythmic theta discharge. Clinical features included initial hyperventilation and frequent gelastic outbursts. A sodium amytal test suggested that major speech localization was in the left hemisphere (but the test was suboptimal). The angiogram performed for the amytal test did not disclose any abnormal displacement of the lateral LSAs or other arteries. Based on this and the three-dimensional (3-D) reconstructions in Fig. 8.4, the LSAs are presumed to be draped on the back side of the lesion and particular care would be necessary in this area. Injury to the LSAs represents the major risk to motor function in this case because the lesion is remote from the internal capsule and corona radiata. The artery of the central sulcus and distal M2 perforators should not be subject to much manipulation. Whereas it is unclear whether speech representation in the insula is of significance, circumnavigating the opercular cortex to reach the lesion and LA carry an unequivocal risk to speech. In this context, awake speech mapping would be of value to determine speech localization with a view to selecting the functionally safest approach. Fig. 8.4 MRI 3-D reconstructions of a patient with a 12-year seizure history and a cavernous angiomas (in green) deep to the limen insula. Venous anatomy is in blue; arterial anatomy is in red. (A–C) Different surgical angles of approach as detailed in the text. In Figs. 8.4 and 8.5, three different angles of approach are illustrated as A, B and C. Approach A is subfrontal and would have the advantage of avoiding manipulation of Broca’s area of the frontal operculum. It is the longest approach, however, and in isolation would not permit adequate visualization of the MCA and the LSAs from the M1 segment in particular. The lesion does abut posterior orbitofrontal cortex, which may contribute to the epileptogenic network. Approach B, an anterior transsylvian approach would allow good microsurgical exposure, but manipulation of Broca’s area is most likely. This author has avoided self-retaining retractors whenever possible for the past 10 years. Direct retraction on any cortex, but especially highly eloquent cortex, can be detrimental both in the short and long term. Lang et al report a case in their insular tumor series where dominant frontoopercular retraction led to speech dysfunction during awake mapping, which was alleviated by removing the retractor. Approach C represents a more direct transinsular approach to the lesion more removed from presumed Broca’s area, but it would be more likely to lead to manipulation of M2 branches, with lesser visualization of the LSAs. A fourth approach (not illustrated) could be a low temporal transamygdalar approach. This approach would avoid opercular cortex, but it would involve removing presumably normal amygdala.
Insular Seizure Semiology
Insular Anatomy and Physiology
Vascular Anatomy and the Insular Lobe
Risk Assessment of Surgical Approaches to the Insula
Lesional Epilepsy Surgery and the Insula
Vascular
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








