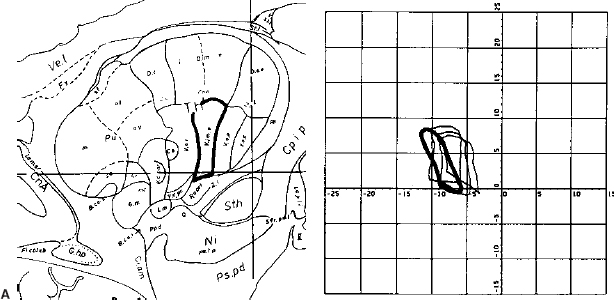
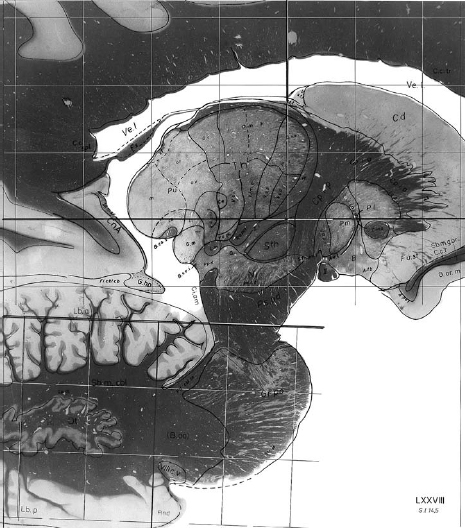
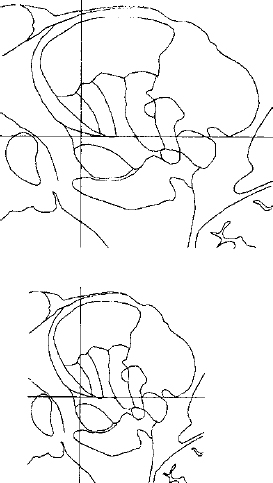
14 One of the fundamental problems inherent in microelectrode recording during functional neurosurgical procedures is that of relating the position of the electrode tip to the neural structure in which the electrode lies. Because it is not possible with present technology to visualize the actual relationship of the microelectrode to the deep nuclei, indirect methods must be used to achieve a successively more accurate approximation of the location of the recording surface of the electrode. Throughout the history of intracranial functional surgery innovative methods have been developed to achieve this correlation between imaging and physiology culminating with modern systems for integrating multiple information sources, including MER. Various strategies have been utilized to relate electrode position to anatomic localization. It may even be debatable as to whether it is useful to relate physiological data to anatomy. After all, it could be argued that targeting for functional procedures should be based more on physiology than on anatomy because the object is to affect the function of the brain rather than the structure. An electrode track represents a very precise map in one dimension (depth); if the electrode is constrained in all other dimensions and reintroduced along this track with high precision, then targeting of the desired cell group can be exquisitely precise. However, there are several advantages to relating the physiology obtained from microelectrode recording with the anatomy of the deep nuclei of the brain. There are some instances where the ability to accurately relate physiology to anatomy becomes very important, even vital. One is when initial targeting passes have failed to correspond precisely to expectations. In this case, the assumed map of the targeted structure may differ significantly enough from the actual anatomy that the surgeon becomes confused and lost. Without a precise map, assumptions are being made regarding the boundaries and shape of the nucleus being targeted. Although experienced surgical neurophysiologists have built up internal “cognitive maps” of many of the more familiar structures, such as the globus pallidus, subthalamic nucleus, and ventral tier nuclei of the thalamus, indications for deep brain stimulation are rapidly expanding to other targets, with unknown electrophysiological characteristics. The ability to relate these recordings to anatomy not only can shorten the learning curve of clinically effective targeting but also can provide a repository of linked anatomical and physiological data, which, if pooled from multiple patients, could allow investigators to gain insights into the overall relationship of anatomy to function. Without some way to relate all of these data to a similar anatomical target, it is difficult to make definite conclusions, whereas with accurate coregistration of targeting between various patients and groups, very powerful statements could perhaps be made regarding optimal targeting parameters. The introduction of human stereotactic surgery in 1947 by Spiegel et al1 ushered in a new era of possibilities for altering the function of the brain. They based their technique of “stereoencephalotomy” on the groundbreaking work of Horsley and Clarke,2 who described a method for overlaying a three-dimensional Cartesian coordinate system onto the brain of a laboratory animal, the macaque monkey. These early investigators realized the necessity of precisely relating the position of the targeting apparatus to particular structures within the brain, which was initially accomplished by aligning brain slices to recognizable and reproducible landmarks on the animal’s skull. Thus was born the first stereotactic atlas. The first human stereotactic atlas was published by Spiegel and Wycis in 1952.3 Their original reference plane, the posterior commissure–pontomedullary sulcus line, was somewhat cumbersome to use and also unreliable. However, this groundbreaking atlas nonetheless provided valuable data on anatomic and radiographic variability as well as postmortem evaluations of targeting accuracy. Talairach et al’s well-known atlas4 introduced, among other innovations, the more reliable anterior commissure–posterior commissure plane. Some of the most widely used atlases have been those developed by Schaltenbrand and colleagues.5,6 These myelin-stained sections, with nuclear boundaries traced on overlays, have guided numerous stereotactic surgeons for over 40 years (Fig. 14–1). However, as with all printed atlases, the variability of individual brains renders even these detailed images an approximation at best, and a deceptive road map at worst. The problem of matching atlas anatomy to that of the patient has been a source of constant study and innovation ever since Spiegel and colleagues’ original work. FIGURE 14–1 Plate 45 from the Schaltenbrand and Wahren stereotactic atlas, with the ventral tier nuclei Voa, Vop, Vim, and Vc delineated on an acetate overlay. This atlas slice is the one most commonly used for targeting the Vim nucleus for the treatment of tremor and thus has served as a template for several studies of thalamic physiology. As interest in stereotactic interventions for movement disorders grew, refinement of targeting techniques was vigorously pursued. In 1966, the American Association of Neurological Surgeons held a special symposium on Parkinson’s disease, at which several localization techniques were discussed. As Spiegel lamented, after nearly 20 years of working to refine the techniques of stereotactic surgery, “a problem not yet completely solved is the individual variability of the co-ordinates of the relevant intracerebral structures.”7 Refinement of targeting by MER was discussed as one way to help compensate for anatomic variability,8,9 but the essential problem remained. In 1966, it was “known that this variation is sufficiently great that discrete placement of an electrode in the exact center of a subcortical target, such as the nucleus ventralis lateralis, is not possible on the basis of stereotactic co-ordinates alone.”10 Van Buren and Borke11 studied this specifically in their atlas of variations of the thalamic nuclei (Fig. 14–2), graphically illustrating the wide differences in nuclear shape, size, and position. Thus, some method was needed to alter the configuration of the atlas to fit the individual patient’s brain. Several different approaches were taken to address this need. Talairach et al4 invented a system to subdivide the thalamus proportionately and thus scale it to the radiographic landmarks, and this method is still in use today. Schaltenbrand’s stereotactic operating room contained elaborate equipment to perform optical projection of multiple scaled imaging modalities onto a screen.12 But it was not until the digital computer was introduced that a rapid, accurate, reproducible method of scaling could be practically utilized. Tasker and his group13 in Toronto reported on a revolutionary method for stretching or shrinking the entire thalamus to fit an individual patient’s AC–PC dimensions, scaling all subnuclei uniformly as based on the Schaltenbrand atlas (Fig. 14–3). By using a computer to carry out a task that would have been impossible by hand, Tasker et al demonstrated the utility of computers to assist in stereotactic surgery. In addition, his description of mapping MER data onto this scaled atlas took the next step toward true integration of stereotactic position with physiology (Fig. 14–4). Tasker et al’s groundbreaking work has been extended and expanded by Nowinski and colleagues,14,15 who have produced a fully scalable atlas, integrating both Schaltenbrand and Talairach atlases and incorporating sophisticated tools for atlas scaling and targeting. Even in the modern era of sophisticated stereotactic interventions, controversy exists regarding the best method of targeting the deep nuclei. Various groups use different criteria for target selection, ranging from integration of imaging, atlases, standard calculated target coordinates, and MER,16 to nearly complete reliance on MER to define the target, using an atlas only as an initial targeting aid.17 Some groups utilize the raw Schaltenbrand and Bailey atlas as an overlay without scaling or other adjustment,18 although this method has many potential inaccuracies, as discussed above. As imaging modalities have been further refined, definition and identification of the deep nuclei have become more reliable. For example, many groups now use direct imaging of the subthalamic nucleus for initial targeting, an accomplishment that is impossible using CT scanning alone. High-field MRI promises to further improve our visualization of targets for deep brain stimulation.19 However, despite the advances in MR imaging, some targets remain invisible. The sub-nuclei of the thalamus may be difficult to define even in histological sections. Even well-visualized nuclei like the subthalamic nucleus can often have indistinct boundaries, making their localization subjective. As our indications for DBS expand, targets in other areas such as the hypothalamus and brainstem will remain ill-defined even at high field strengths. It is thus probable that atlases will be useful for the foreseeable future. However, there are several limitations inherent in atlases such as the Schaltenbrand atlases. They are not internally self-consistent, being composed by necessity of sections from different cadaver specimens. The nuclear boundaries therefore do not correspond in all three planes. In addition, the atlas is not truly three-dimensional, because the distance between slices is relatively large and varies from slice to slice. Thus, there has existed a need for a truly three-dimensional atlas that would correspond exactly to an individual patient’s brain, deformed precisely to fit rather than scaled approximately. Several groups have worked successfully on producing a deformable atlas.20,21 One such atlas, developed by Talairach’s group, was defined by tracing (“segmenting”) nuclear boundaries on successive slices of a volumetric, high-resolution T1 MRI dataset from a normal volunteer. These three-dimensional boundaries form the “atlas” data that are used to generate specific contours unique to a particular patient. Any T1-weighted MR imaging scan can then be deformed to fit the “atlas” MRI using a sophisticated computer algorithm that relies on a fluid dynamics–based model of intensity matching to stretch, shrink, and warp the patient’s brain images to fit those of the atlas brain.22 This mathematical deformation creates a vector field that describes the way each point on the patient’s scan has been deformed to fit the atlas scan. If this vector field is then applied to the traced outlines of the deep nuclei, these outlines can be deformed back from the atlas MRI to the patient’s MRI, giving a patient-specific representation of the atlas nuclei in true three-dimensional form. Initial clinical tests of this atlas have been extremely promising, demonstrating a high degree of localization accuracy.23 FIGURE 14–3 Tasker’s illustration of computerized stretching (upper) and shrinking (lower) of the 13.5 mm lateral slice from the Schaltenbrand and Bailey atlas, the outlines of which correspond to plate 45 of the Schaltenbrand and Wahren atlas, as shown in previous figures.
Integration of Stereotactic Position with MER
JAMIE M. HENDERSON
Atlases: Road Maps for MER
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Neupsy Key
Fastest Neupsy Insight Engine