Condition
Synopsis of clinical condition and monitoring
Synopsis of management
Pre-procedure renal protection
Absolute increase by 0.5 mg/dl or 25 % relative increase from baseline seen 2–7 days after contrast exposure
If ≥2 risk factors for contrast nephropathy, NAC 600–1,200 mg PO Q6 h× 4 doses pre-procedure and/or 1 ml/kg/h normal saline or sodium bicarbonate 6–12 h pre- and post-procedure
Vascular access site complications
Local hematoma
Retroperitoneal hematoma
Pseudoaneurysm
Arteriovenous fistula
Local growth; margins should be demarcated
Flank and abdominal pain, painful and cold extremity. Potentially life threatening due to acute anemia and shock
Ecchymosis, pulsatile palpable mass and presence of bruit. Untreated source of emboli and infection and potential rupture
Rare iatrogenic communication between femoral artery and vein
Most resolve spontaneously; in rare cases chronic AVF may cause cardiac failure, limb edema, or degeneration of the artery
Local compression
Frequent marking and serial checks
Frequent hemoglobin checks
CT abdomen; aggressive management of shock
Progressive worsening—consider blood transfusion, reversal of anticoagulation, surgical/angiographic exploration of arteriotomy site
Duplex ultrasound or CT/MR angiography in some cases
If small (<2 cm), observe. Larger pseudoaneurysm could be treated using ultrasound-guided compression or rarely open surgical repair
Serial duplex ultrasound. USGC or endovascular/open surgical repair for chronic AVF
Stroke thrombectomy
Blood pressure management
Intracranial hemorrhage (ICH)
Increased ICP management
Herniation or ICP crisis
Frequent neurological examination
Signs of increased intracranial pressure with change in neurological exam
Risk factors: onset-to-reperfusion time >270 mins, initial larger stroke >1/3 of MCA, or severe deficits at presentation (NIHSS >20)
Sustained ICP >20 mmHg with neuro worsening
Sustained ICP >25 mmHg for prolonged period
Unilateral pupil dilation
CT scan with or without CT angiogram if exam changes
Complete recanalization—SBP range 120–140 mmHg
Partial recanalization—BP range more liberal up to 180/105
Prompt reversal of anticoagulation; consider checking fibrinogen levels with FFP ± platelet transfusion
Consider prophylactic hypernatremia
Neurosurgery consultation for potential decompression or EVD placement
Elevation of bed to 30°
Mannitol (average 1 g/kg) Q6 h +/− hypertonic saline
Monitor osmolar gap and renal function
Hyperventilate to maintain PaCO2 ~ 30 mmHg
Bolus mannitol 1–1.5 g/kg or 23.4 % NaCl 0.67 cc/kg
Subarachnoid hemorrhage
Blood pressure management
Vasospasm
Frequent neurological examination
Commonly seen 3–21 days post-aSAH
Neurological deterioration
Incidence of rebleed is 5–10 % in first 72 h
Maintain SBP <160 and MAP <110 prior to securing aneurysm
Daily transcranial Doppler (TCD); maintain euvolemia
Diagnostic angiogram or CT/MR angiography
Induce hypertension using boluses with or without vasopressor
Sustained vasospasm intra-arterial vasodilator and/or angioplasty
Carotid artery stenting
Cardiovascular complications
Bradycardia
Myocardial infarction
Ischemic stroke
Intracerebral hemorrhage
Cerebral hyperperfusion syndrome
Frequent neurological examination
Avoid sedation and pain medication, may mask symptoms
Persistent heart rate below 60 beats per minute
Risk of periprocedural MI high (~1.3 %)
Periprocedural risk is ~3.5 %
New neurological deficit
Ipsilateral headache, seizure, and transient neurological deficit in the absence of ischemic or hemorrhagic injury
Dual antiplatelet therapy should be started in all patients pre-procedure
Atropine IV 0.5–0.75 mg if symptomatic
Continue pre-procedure beta-blocker at reduced dose
Start or continue statins
Optimize electrolytes K >4, Mg >2, calcium >8
Cardiology consult if new onset arrhythmia and troponin leak
CT brain rules out ICH
CT angiography or conventional angiography to investigate in-stent thrombosis versus intraluminal occlusive
Consider emergent intra-arterial tPA or mechanical thrombectomy
Maintain blood pressure close to normal
Symptomatic headache therapy
Symptomatic seizure therapy with antiepileptics
Intracranial embolization procedures
Hemorrhagic complications
Ischemic complications
Seizure
Frequent neurological examination
Neurological deterioration
Iatrogenic secondary to catheter-induced emboli
Common ~8 %
Blood pressure regulation is extremely important post-procedurally
All patients should be kept normotensive and euvolemic
CT brain to rule out ICH
Prevented with systemic heparinization
First-line antiepileptic
Pre-procedural Care
Fasting
Pre-procedural fasting (NPO or nulla per os) reduces the risk of gastric regurgitation. Usually, patients are kept “NPO after midnight” in anticipation of a planned procedure during the daytime. Care must be taken to avoid starvation, dehydration, and electrolyte imbalance in this situation. The American Society of Anesthesiologists practice guidelines recommend abstaining from clear liquids (should not contain alcohol) for at least 2 h prior to elective procedures, 6 h or more from light meals, and ≥8 h from larger meals with high fat content [1]. These recommendations are meant for healthy individuals only and may need to be modified for patients with conditions that affect gastric emptying (as in diabetes, gastroesophageal reflux disease, or enteral feeding) or those who are at high risk for regurgitation or tracheobronchial aspiration.
Hydration
Patients undergoing neurointerventional procedures that have intact thirst mechanisms may be able to maintain euvolemia by ingesting fluids while in the intensive care unit. Those with neurological injuries that impair swallowing, with thirst, or who are intubated should be administered fluids in the event of adequate renal function at the rate of 30 ml/kg/day. Usually crystalloids are sufficient. Colloids may occasionally be used for rapid intravascular volume replacement, although they are much more expensive and may cause unwarranted side effects [2].
Renal Protection
Patients undergoing angiograms, especially those with renal insufficiency, diabetes, and congestive heart failure, are at risk for contrast nephropathy due to regional hypoxia or acute tubular necrosis. It is usually diagnosed based on an absolute increase of serum creatinine of 0.5 mg/dl or by a 25 % relative increase from the baseline value. The rise is usually seen 2–7 days after contrast exposure and may persist for 2 weeks or longer. This can be a serious event, resulting in prolonged hospitalization, dialysis, or even death. The risk of contrast nephropathy appears to be smaller with iso-osmolar, dimeric, nonionic contrast agents than low-osmolar, nonionic, monomeric contrast agents [3]. If the patient requires a dye-based study, risk factors should be considered. If ≥2 risk factors for contrast nephropathy are present, nephroprotection using N-acetylcysteine 600–1,200 mg orally for four doses periprocedurally should be considered. Otherwise, hydration with 1 ml/kg/h of intravenous normal saline for 6–12 h before and after the procedure should be administered while minimizing the use of contrast dye during the procedure. Sodium bicarbonate likely has a similar protective effect to normal saline, although some believe its alkalinizing action on the renal tubular fluid provides additional protection [4].
Post-procedural Care
Vascular Access Site Complications
Vascular access site (VAS) care is important in post-procedural care, and neurointensivists should be familiar with the prevention and management of related complications. The rate of percutaneous VAS complications due to neurointervention is reported to be between 0.3 and 4.2 % [5, 6]. Improved closure devices have contributed to decreased morbidity and mortality in recent years [7]. A detailed hand-off communication [8] helps in early detection and prompt management [9]. Femoral access is commonly employed in neurointerventional cases, and related complications will be discussed.
Hematoma
A localized hematoma remains the most common complication in any endovascular procedure. The adverse consequences range from minimal to life threatening if retroperitoneal space is involved. A high puncture level above the inguinal ligament is an important risk factor for the development of retroperitoneal hematoma.
A hematoma will cause pain and swelling, while a retroperitoneal hematoma may result in flank/abdominal pain, a painful/cold limb, loss of peripheral pulses, acute anemia, and hemorrhagic shock in extreme cases. Management with local compression, serial groin/flank checks marking hematoma growth, and frequent follow-up of hemoglobin is the first line of treatment. There should be a low threshold to get CT of the abdomen and pelvis in patients with falling hematocrit and physical signs consistent with shock. Progressive blood loss and hypovolemic shock necessitate reversal of anticoagulation, blood transfusion, and aggressive management of shock. Continued bleeding with evidence of retroperitoneal bleeding requiring multiple transfusions may necessitate surgical/endovascular management to explore the arteriotomy site for active bleeding and repair.
Long-term complications of these hematomas include compressive femoral neuropathy/lumbar plexopathy causing weakness, numbness, and pain.
Pseudoaneurysm
A pseudoaneurysm is a contained vascular rupture (hematoma) within the elements of the surrounding tissue at the arteriotomy site that creates turbulent flow between the vessel and soft tissue space. Diagnosis is suspected based on a groin examination that shows ecchymosis, a pulsatile palpable mass, and/or bruit. If left untreated, the pseudoaneurysm can serve as a source of emboli, infection, or rupture [10]. Duplex ultrasound shows a hypoechoic region, either lobulated or cystic, adjacent to the artery with color flow map that demonstrates turbulent, swirling flow (“yin-yang sign”) [11]. Obese patients may need CT or MR angiography for diagnosis. Retroperitoneal extension may be investigated using a CT of the abdomen to identify the arterial injuries.
Management and treatment of a pseudoaneurysm depends on the size, location, and progression of the lesion. Smaller pseudoaneurysms (<2 cm) can be observed with frequent Doppler exams. Larger pseudoaneurysms are treated using ultrasound-guided compression (USGC), ultrasound-guided thrombin injection (USGTI), or less commonly open surgical repair.
Arteriovenous fistula (AVF)
Iatrogenic fistulas (communication between femoral artery and vein) are a rare VAS complication. Most cases resolve spontaneously, but rarely long-standing fistula may give rise to cardiac failure, limb edema, or degeneration of the artery. Treatment should be pursued using USGC or endovascular or open surgical repair.
Infection
Infection is a low-frequency complication in percutaneous interventions (including diagnostics, angioplasty, stenting, and/or embolization procedures). There is no need to use prophylactic antibiotics [12].
Stroke Thrombectomy (See Chap. 7)
Only about 7 % of acute ischemic stroke patients receive intravenous (IV) thrombolysis. Clinical recovery depends on recanalization of the affected blood vessel(s) but occurs in only 20–30 % of large artery occlusions treated with IV tPA [13, 14]. Mechanical thrombectomy with stent retriever devices has better rates of recanalization and fewer complications (e.g., intracerebral hemorrhage) than other methods even though its clinical efficacy has not yet been fully proven [15–17]. While some trials (e.g., REVASCAT) are attempting to establish the clinical utility of mechanical thrombectomy in prespecified settings, it is currently offered on a case-by-case basis to eligible patients. Medical management of such patients in the neurocritical care unit remains an important task for the neurointensivist.
Blood pressure management
Blood pressure (BP) should be frequently monitored along with the neurologic examination for the first 24 h in patients receiving mechanical thrombectomy. BP parameters are similar to management following IV thrombolysis, although experts suggest different titration strategies based on the extent of recanalization [18, 19]. Sudden changes in BP with worsening of neurological examination should immediately be investigated for spontaneous intracranial hemorrhage. In the event of full recanalization, BP should be kept in the systolic 120–140 mmHg range to minimize the risk of reperfusion hemorrhage. Those receiving mechanical thrombectomy alone with only partial recanalization may benefit from a higher BP target—up to 180/105 mmHg.
Intracranial hemorrhage
Small volume intracranial hemorrhage (ICH) after thrombolysis is frequently seen on follow-up imaging studies (Fig. 3.1). This may be a marker of early successful recanalization that has been shown to be associated with reduced infarct size and improved clinical outcome [20]. On the other hand, deterioration in neurological examination with ICH or symptomatic ICH (sICH) is a feared complication that results in worsened outcomes and increased mortality. This risk may increase with longer onset-to-reperfusion times (>270 min) [21]. In addition, more severe deficits at presentation (NIHSS > 20), the presence of cerebral edema on initial imaging, involvement of >1/3 of the MCA territory, as well as deviation from thrombolysis protocols may increase the risk of sICH. Guidelines regarding management of sICH following thrombolysis/thrombectomy are currently lacking, and empirical treatment is done based on the clinician’s judgment. Some strategies are based on fibrinogen levels—if <100 mg/dl, cryoprecipitate is administered at 0.15 U/kg. Alternatively, fresh frozen plasma along with platelets (6–8 U, if platelet dysfunction is suspected) is relied upon at some centers [22]. A neurosurgical consultation should be obtained for potential decompression or clot evacuation in the event of a life-threatening decline, while remaining vigilant for secondary cardiorespiratory failure. Seizure prophylaxis in the event of sICH is not generally recommended, although this area also is lacking in high-level evidence.
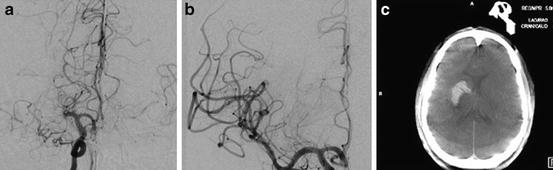
Fig. 3.1
Reperfusion hemorrhage following successful treatment of MCA occlusion. Cerebral angiogram shows a MCA-M1 occlusion (a), which was successfully recanalized (b). Post-thrombectomy computed tomography (CT) shows intracerebral hemorrhage (c)
Intracranial Pressure (ICP) management
Large hemispheric infarcts complicated by hemorrhagic conversion or cerebral edema can result in elevated ICP. There is no benefit in routine ICP monitoring as patients may develop pupillary abnormalities and signs of brainstem compression even with normal ICP values [23]. Patients with high NIHSS (>15) on presentation and large territorial involvement due to a large vessel occlusion are at risk for developing malignant MCA infarction.
While sICH generally occurs in the first 24 h after presentation, cytotoxic edema and ICP elevation often peak 3–4 days after the initial injury. Patients with large hemispheric infarcts that involve >50 % MCA territory with greater than 66 % perfusion deficit are at risk of developing life-threatening malignant brain edema within 24 h [24]. Hemicraniectomy provides definitive treatment for malignant brain edema while medical management is at best temporizing in such situations.
Hemicraniectomy
Decompressive hemicraniectomy (DHC) is the definitive treatment for malignant cerebral edema due to middle cerebral artery (MCA) infarcts. It normalizes the intracranial pressure, improves the cerebral blood flow, and reverses the herniation impacting the contralateral hemisphere and midline structures.
The pooled analysis of three European hemicraniectomy trials proved the efficacy of DHC performed up to 48 h from the onset of symptoms. A reduction in mortality was seen in patients up to 60 years of age (number needed to treat =2) [25]. These trials included patients with MCA strokes on either side. Similar benefits may be seen in patients up to 70 years of age based on the findings from the recently completed DESTINY II trial [26]. The impact of DHC on functional outcomes was not as robust in this age group. Despite this fact, patients reported satisfaction with their quality of life even in the setting of a high degree of physical disability and depression [27].
Patients benefit from DHC irrespective of the hemisphere involved. The benefit is most pronounced if surgery is performed within 24 h. It is unclear whether the benefit would persist if surgery were performed beyond 48 h. Since not all large territory MCA infarctions lead to malignant edema requiring DHC, vigilant neuromonitoring is needed. Infarct volumes >145 cm3 at 14 h seen on magnetic resonance diffusion-weighted imaging were shown to have 100 % sensitivity and 94 % specificity in predicting malignant edema [28]. Infarct volumes >82 cm3 at 6 h have 98 % specificity but lower (52 %) sensitivity [28].
An adequate DHC should be 14 cm in anteroposterior diameter as well as 9 cm in vertical diameter (Fig. 3.2). The neurointensivist should watch for the complications of an undersized craniectomy that may result in mushrooming herniation of the brain through the craniectomy leading to further ischemia. Additional care should be paid to the development of subdural hygromas, hydrocephalus, and hemorrhage at the craniectomy site as well as any signs of infection at the site of surgery.
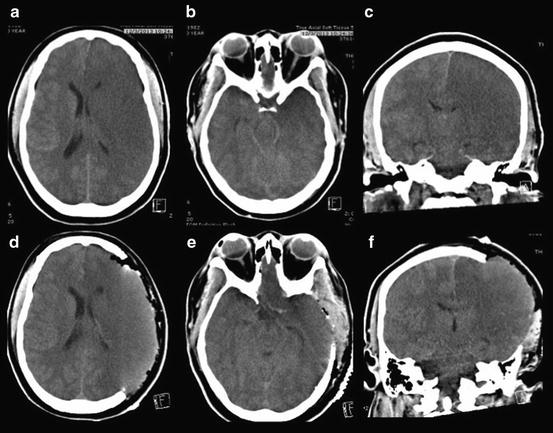
Fig. 3.2
Malignant MCA infarction requiring decompressive craniectomy. Computed tomography (CT) shows malignant right middle cerebral artery (MCA) infarction with extension into the temporal lobe (a–c). Post-surgical resolution of subfalcine herniation with overlying scalp expanded to accommodate expanding brain (d–f)
Medical
The first priority in ICP management is ensuring adequate airway, breathing, and circulation. Other medical interventions in the management of ICP in malignant MCA infarction are temporizing measures. Mortality can be up to 70 % when relying upon medical management only in this population. Medical management of ICP includes elevation of bed to 30°; use of mannitol (average 1 g/kg) every 6 h while maintaining euvolemia, monitoring osmolar gap (should reconsider mannitol if gap >15) and renal function; hypertonic saline; and intubation with hyperventilation. There is currently insufficient evidence to advocate the use of prophylactic hypernatremia in preventing the development of cerebral edema following ischemic stroke [29].
Antithrombotic use post-thrombectomy
Initiation of anticoagulation and/or antiplatelet agents is an individualized decision post-thrombectomy given the absence of formal guidelines. Initial studies and clinical trials (NINDS and PROACT II) excluded patients with infarct volume >1/3 MCA territory due to concerns of hemorrhagic reperfusion injury. In general if reperfusion hemorrhage has occurred, antithrombotic therapy should be held for 1–4 weeks.
Subarachnoid Hemorrhage (See Chap. 11)
Aneurysmal subarachnoid hemorrhage (aSAH) patients should be cared for in a high-volume center. The neurointensivist acts as the linchpin to ensure appropriate care in this high morbidity and mortality disease. Various organ systems may be affected through the course of the patient’s presentation. Knowledge of these conditions is required to provide the best possible care.
BP management
Aneurysmal rupture is associated with a sympathetic surge and resultant increase in blood pressure. The incidence of rebleeding is 5–10 % in the first 72 h. The exact blood pressure beyond which the risk of rebleeding significantly rises is not clear; however, systolic blood pressures up to 160 mmHg or mean blood pressure up to 110 mmHg is generally acceptable. Short-acting BP medications (e.g., labetalol or hydralazine) may be used intermittently or continuously (e.g., nicardipine). Premorbid blood pressure should be taken into consideration while hypotension should be avoided. Hypertension that is accompanied by bradycardia should raise concern for increased intracranial pressure and may require osmotic therapy to treat cerebral edema or extraventricular diversion of cerebrospinal fluid if hydrocephalus is diagnosed.
BP is commonly elevated in the acute phase following aSAH. After the aneurysm is secured, strict BP control is not required. BP goals should be relaxed to allow for cerebral perfusion. Strict BP control may mask the early warning signs of vasospasm during which BP typically increases. BP should be titrated in the event of signs and symptoms of end-organ damage.
Vasospasm
Arterial narrowing or vasospasm as evidenced on angiography is seen between 3 and 21 days post-aneurysmal rupture with the peak being around 7–10 days. It is seen in two-thirds of patients presenting with aSAH, and in approximately 30 % of such cases, arterial vasospasm results in focal neurological deficits also known as delayed cerebral ischemia (DCI). If not treated appropriately, DCI can result in cerebral infarction—a feared complication that can greatly affect the long-term outcome. Vasospasm risk increases with the thickness of SAH as measured by the modified Fisher scale [30]. Nimodipine (60 mg every 4 h), a dihydropyridine calcium channel antagonist, is the only agent shown to reduce cerebral ischemia and mortality when used prophylactically. It is used for 21 days in aSAH. Sometimes due to its hypotensive effect, it may need to be given at a reduced dose but greater frequency (30 mg every 2 h).
In the event of neurological worsening, potential confounders (fever, hypotension, hyponatremia, seizures, etc.) should be ruled out. In parallel, vasospasm should be investigated using catheter angiography. While catheter angiography is the preferred modality in cases at high risk for vasospasm where intra-arterial intervention may be required, CT/MR angiograms with/without perfusion maybe reasonable alternatives in select situations. CT/MR angiograms are less sensitive in detecting mild–moderate vasospasm compared to catheter angiography. While planning for a catheter angiogram, hemodynamic augmentation should be attempted using a fluid bolus with/without a vasopressor (Neo-Synephrine or norepinephrine) in order to up-titrate mean arterial pressure by 15–20 %. Occasionally, an inotropic agent may be useful in this setting. If vasospasm on angiogram is diagnosed, it may require treatment with intra-arterial vasodilator agents or angioplasty. Only the induced hypertension component of the conventional “triple H” therapy should be used as hypervolemia and hemodilution do not improve cerebral blood flow or oxygen delivery and may even cause cardiopulmonary complications.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








