Fig. 1
Left image: Side view of rhesus monkey brain with eye underneath frontal lobe for orientation. The tail of the red line is over primary visual cortex (V1), the body overlies the visual areas in the temporal representing the cascade of the ventral visual stream, and the arrow head suggests that information goes underneath to medial temporal lobe structures. The turquoise region is area TE, thought to be a final step for visual pattern recognition. Right image: bottom view of brain, now oriented with frontal cortex at the top and primary visual cortex at the bottom. The purple highlights the classical orbitofrontal cortex (OFC), the blue rhinal cortex (Rh) which includes perirhinal and entorhinal cortices, and the turquoise the lateral inferior temporal cortex area TE. The long red arrow is placed to match the one on the right image, and the short red arrow indicates the existence of the reciprocal connections between OFC and Rh cortex [1]
To study how information is transformed as it passes from one brain region during reward evaluation, we have studied the contributions of the rhinal cortex in the medial temporal lobe and the orbitofrontal cortex in the monkey (Fig. 1). These regions are known to have reciprocal intrahemispheric connections, and ablations of them are followed by deficits in normal reward related behavior. Monkeys discriminate less well between rewards of different sizes after bilateral removals of orbitofrontal cortex than before the removals. After bilateral removals of rhinal cortex monkeys have a severe deficit in distinguishing among rewards also [4]. This set of results led us to ask whether the ability to distinguish among different reward sizes relied on communication between these brain regions.
We used a disconnection experiment [5]. In this study the rhinal cortex on one side and the orbitofrontal cortex on the other were removed. We then compared these monkeys to control monkeys, to monkeys with bilateral removals of rhinal cortex and to monkeys with bilateral removals orbitofrontal cortex. We used a very simple behavior in which the monkeys just touched and released a touch sensitive bar. For each set of 25 trials, a single size reward was delivered, and after each set of 25 trials, the reward side was changed randomly, with the set of rewards being 1, 2, 4, and 8 drops of juice. We measured the ‘release interval’, that is, the length of time from touch to release.
The control monkeys react faster for larger rewards (Fig. 2). The monkeys with bilateral OFC removals show a similar tendency, although they react faster overall, a result similar to that seen when visual cues, rather than relying on the local history (here the monkeys know what reward is forthcoming because, overall, the next reward will be the same as the preceding one). In both the case with bilateral rhinal lesions and the rhinal-orbitofrontal disconnection, the monkeys do not change the performance with which they carry out the behavior. They act as if they unable to distinguish one reward from another.
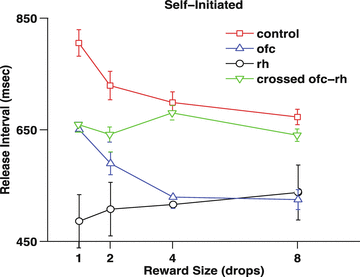

Fig. 2
Self-initiated bar pressing task. The monkey received a reward for each bar press-release sequence. The reward size was constant for 25 press-release sets, and then randomly changed to another reward size, chosen from 1, 2, 4, & 8 drop rewards. The release interval is the length of time from press to release. The results are from different sets of 4 monkeys/group. The monkeys with bilateral removal of rhinal cortex, and with the crossed rhinal-orbitofrontal removal appear to be insensitive to the difference in reward size
These results seem to show that rhinal cortex must communicate with orbitofrontal for normal assessment of reward size. Our results show that the normal monkeys are sensitive to the contextual relation among the available rewards. Two processes need to occur for this to happen. First, there must be a value representation of different rewards, and second, the relative value scale needs to be remembered. Based on a considerable amount of work on OFC [6–8] and Rh we hypothesize that the OFC is critical for encoding reward value rhinal cortex must interact for the normal representation of relative reward values [9–12]. Our hope is that this OFC-Rh system provides a good substrate for studying how information is transformed as it passes between brain regions with different specialization.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






