Chapter 166 Interspinous Bumpers
Lumbar stenosis is defined as the reduction in the diameter of the spinal canal, lateral recess, and/or neural foramina. Stenosis is most frequently a sequela of the degenerative process of aging, but recently genetic factors have been demonstrated to play a significant role.1–3 The degenerative changes of disc desiccation with anular bulging, osteophyte formation, ligamentum hypertrophy, facet hypertrophy, and/or facet joint cyst all contribute to reducing the space available to the cauda equina and causing the classic symptoms of neurogenic claudications. In particular, compression and ischemia of nerve roots are the main source for this type of pain.4–6
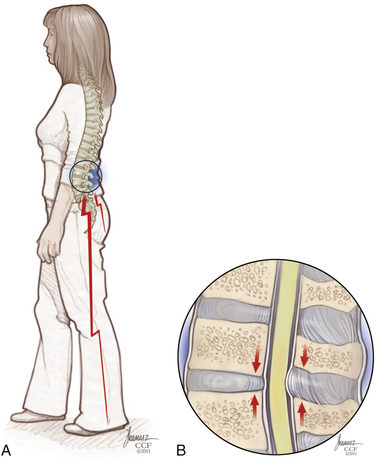
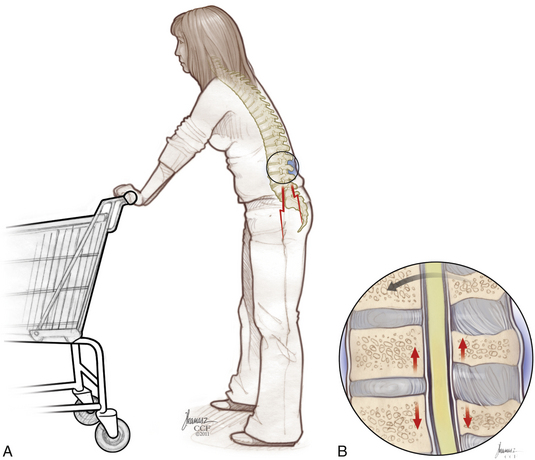
Patients with the classic presentation of intermittent neurogenic claudication have buttock and leg pain when they stand and walk and have relief of symptoms when they flex forward, as when pushing a shopping cart, or when they sit or lie down (Figs. 166-1 and 166-2). This results mainly from the buckling of the ligamentum flavum and a decrease in the size of the central canal, subarticular region, and foraminal area with standing and walking.
The presence of canal stenosis on radiographic imaging does not itself define the syndrome, as there is a poor correlation between the degree of stenosis and the severity of symptoms.7,8
Interspinous Spacers: Indications and Contraindications
It should be noted that in the United States the primary use of these interspinous devices is for the treatment or stabilization of the lumbar spine to correct neurogenic claudication caused by canal stenosis. There is biomechanical evidence of unloading the facet joints and disc space,9 which theoretically may limit back pain associated with a degenerative lumbar segment. In other areas of the world, these devices are used primarily to treat lumbar axial pain, as opposed to neurogenic claudication.
Wallis Interspinous Spacer
In 1986 Sénégas10 developed the first lumbar interspinous implant designed to stiffen unstable operated degenerative segments without eliminating mobility. At first called the Mechanical Normalization System, it included a titanium interspinous blocker and an artificial ligament made of Dacron.
Between 1988 and 1993, more than 300 patients were treated for degenerative conditions with this first-generation device. It was concluded that many patients demonstrated significant resolution of residual low-back pain.11
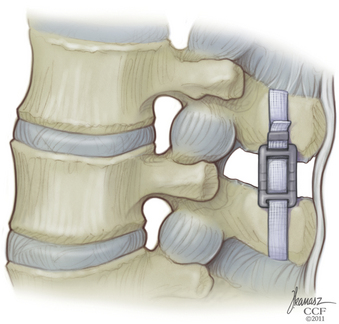
The Wallis System (Abbott Spine, S.A., Bordeaux, France) was the second-generation interspinous spacer. The crucial change was in material properties. The Wallis spacer is made of polyetheretherketone (PEEK), a strong, completely radiolucent material compatible with MRI. This plastic-like polymer is more elastic, which reduces the risk of spinous process stress fractures. In addition, the Wallis device has notches that fit the physiologic shape of the lumbar spine, which minimizes the need for bone resection and avoids constraint on bone.
Surgical Technique
The surgical technique for the Wallis interspinous spacer was initially described by Sénégas.12 The procedure is typically performed under general anesthesia with the patient in a prone position on a radiolucent operating table. The patient’s lumbar midline should be marked before he or she is positioned. A neutral position of physiologic lumbar lordosis is best to optimize the effect of the implant.
The supraspinous ligament is returned to its original position and reinserted onto each spinous process with a single silk suture passed through a hole made in the spinous process (Fig. 166-3).
X-STOP Interspinous Spacer
The X-STOP (Kyphon, Medtronic, Sunnyvale, CA) interspinous process decompression (IPD) device is a two-component oval titanium implant that fits between the spinous processes of the lumbar spine. A newer version has a PEEK sleeve over the titanium insert. Several clinical studies have shown the efficacy of the X-STOP device in treating neurogenic claudication secondary to lumbar stenosis.13–16
Surgical Technique
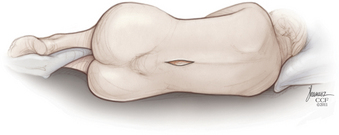
The implant procedure for this device can be done after induction of light sedation or general anesthesia. The patient is placed on a radiolucent table in a right lateral decubitus position; afterward the patient is asked to flex or assume a knee-chest position (Fig. 166-4). The level to be treated is identified by fluoroscopy; localization is at the interspinous level, not the disc space level.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree