54 Interspinous Spacers for Minimally Invasive Treatment of Dynamic Spinal Stenosis and Low Back Pain
KEY POINTS
 It increases the size and areas of the spinal canal as well as of the subarticular zones and the foramen and thus has an indirect “decompression” effect on neural structure.
It increases the size and areas of the spinal canal as well as of the subarticular zones and the foramen and thus has an indirect “decompression” effect on neural structure. It unloads the facet joints as well as the posterior part of the disc and thus has a potential effect on low back pain arising from pathologic load pattern on these anatomical structures. The different implants that are currently on the market or in clinical studies provide these biomechanical effects. They can be categorized in two groups:
It unloads the facet joints as well as the posterior part of the disc and thus has a potential effect on low back pain arising from pathologic load pattern on these anatomical structures. The different implants that are currently on the market or in clinical studies provide these biomechanical effects. They can be categorized in two groups: Nonstabilizing devices used for primary treatment of dynamic spinal stenosis and low back pain (“extension stoppers”)
Nonstabilizing devices used for primary treatment of dynamic spinal stenosis and low back pain (“extension stoppers”)Introduction: Interspinous Spacers – How Do They Work?
Indirect enlargement of the spinal canal through interspinous distraction devices has become popular for the treatment of dynamic spinal canal stenosis of the lumbar spine.1–5 Biomechanical data acquired with the first implant on the market (X-Stop, Medtronic, Memphis, TN, USA), could show that interspinous distraction induces segmental slight flexion, reduces segmental lordosis, and limits extension.6 Thus, the spinal canal and neural foramen areas and diameters are enlarged.7 These findings are considered to be the most important primary effects that justify the clinical use of the device for the treatment of dynamic spinal stenosis. Randomized controlled trials could confirm the therapeutic efficiency and proved that the implantation of an interspinous spacer leads to clinical results superior to conservative treatment.5
In ex vivo experiments it could also be demonstrated that interspinous distraction can lead to a significant unloading of the facet joints8,9 and the posterior annulus fibrosus, as well as the nucleus pulposus in neutral position and predominantly in extension.8,10,11 Kinematics of the adjacent segments seem not to be affected.6,12
The “Extension Stoppers”
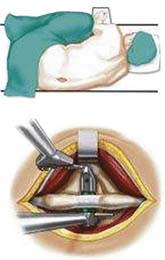
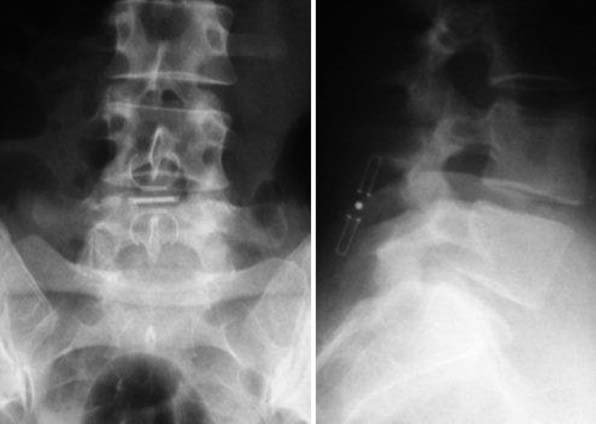
X – Stop (Medtronic) (Figure 54-1)
The main indication is neurogenic claudication with leg/buttock pain due to dynamic degenerative lumbar spinal stenosis, which is relieved upon flexion of the lumbar spine.5
Surgical Technique
It is implanted through a posterior approach. The patient is in a prone position. The dorsolumbar fascia is split on both sides of the spinous processes, the paravertebral muscles are retracted, and the interspinous ligament is pierced. The spinous processes are then actively distracted with a distraction forceps and the X-Stop is implanted from one side. The wing on the contralateral side is then attached (Figure 54-2).
Results
In a randomized controlled trial, it could be shown that the results of the treatment of dynamic spinal stenosis are superior to those in conservative therapy .1,5,13 Whereas in initial reports its usefulness was also documented for degenerative spondylolisthesis not greater than grade I, recent data could not confirm this.14
Summary
Although the implantation of the X-Stop device is claimed to be minimally invasive, it occasionally requires a larger skin incision and a wider bilateral muscular dissection as compared to modern microsurgical direct decompression techniques.15,16 Due to the iatrogenic alteration of the dorsolumbar fascia and the paraspinal muscles, it also cannot be considered as a treatment option for discogenic or arthrogenic low back pain. Moreover, bisegmental or multilevel implantations require larger surgical approaches.
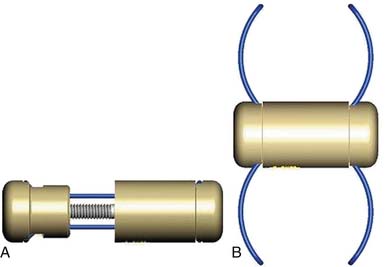
InSpace (Synthes, Paoli, PA, USA) (Figure 54-3)
In order to solve the problem of invasiveness, a new cylindrically shaped PEEK interspinous implant with a central titanium screw and four wings that can be deployed once the implant is placed into the interspinous space, has been presented recently. Biomechnical tests have shown that the implant effectively reduces extension without affecting lateral bending of the segment.17,18 Cyclic loading tests have shown that the functionality of the implant is preserved and that the integrity of anatomic structures is not impaired through 15,000 loading cycles.19,20 The effects are thus comparable to the ones described for the X-Stop implant.
The indications are also identical with those described for X-Stop. There are preliminary reports on its potential usefulness for the treatment of discogenic and/or arthrogenic low back pain.21,22
Surgical Technique
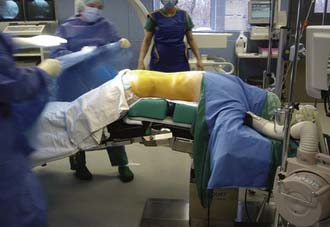
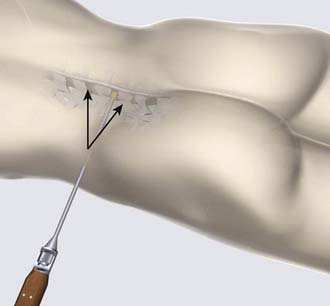
The surgical procedure can be performed under local or general anesthesia. The patient is placed in a prone position on a flat soft-frame on an adjustable operating table or on a Wilson frame. Passive distraction of the interspinous space is achieved and adjusted by tilting the foot end of the surgical table until maximum “opening” of the interspinous space is reached (Figure 54-4). The implant is placed through a lateral percutaneous approach (Figure 54-5). Piercing of the interspinous ligament is performed with a K-wire; enlargement of the interspinous space is achieved with blunt distractors of increasing sizes. After removal of the distractors, the implant can be introduced through an application sleeve and the implant wings are deployed under AP fluoroscopic control. Once the wings are deployed completely, the implant is uncoupled from the implant holder, which, together with the application sleeve, is then removed en bloc, leaving the implant in place (Figure 54-6).
Results
The first operation worldwide was performed on March 15, 2006. Preliminary results in 41 patients show a good reduction of pain level as well as of the Oswestry Disability Index in patients with low back pain as well as in patients with dynamic degenerative lumbar spinal stensosis.22
Summary
InSpace is significantly less invasive as compared to all other extension stoppers currently on the market. The average intraoperative blood loss was less than 5 cc. Surgical time for a single level is usually less than 15 minutes in uncomplicated cases. There were no clinically relevant intraoperative complications. Other advantages of this lateral approach are the short learning curve and no significant blood loss. It can be performed as an outpatient procedure. Postoperative magnetic resonance imaging does not show any evidence of muscular damage or hematoma. The technical limitations are at L5-S1 or in patients with a high iliac crest, due to the angulation required to access the interspinous space. There are still few clinical data available. The implant is currently used in a prospective randomized controlled IDE trial in the United States for the treatment of dynamic lumbar spinal stenosis.
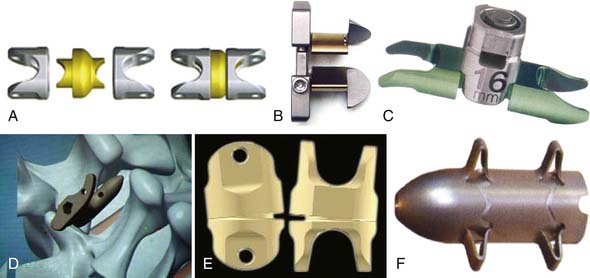
Other Implant Types (Figure 54-7)
Coflex (Paradigm Spine, New York, NY, USA) (Figure 54-8)
This is a U-shaped titanium implant with two bendable wings on its cranial and caudal parts. The Coflex is a dynamic extension stopper that acts like a spring in such a way that extension leads to an elastic compression of the “U” (Figure 54-9). It is either used as an adjunct to open decompression in spinal stenosis cases to unload the facet joints and to “keep the spinal canal open,” or following discectomy to “protect” the disc from excessive load. It thus represents a low back pain treatment concept, i.e., a dynamic stabilization to reduce the load on the facet joints and/or the disc space, and/or to keep the spinal canal “open” by interspinous distraction following decompression procedures.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree