Interventions in the Pediatric Sleep Laboratory
Carla A. Evans
Carol Wood
Carla Uy
Karen Waters
LEARNING OBJECTIVES
On completion of this chapter, the reader should be able to:
1. Discuss the nuances of continuous positive airway pressure titration in children.
2. Explain the importance of monitoring carbon dioxide when titrating oxygen in children.
3. Describe when and how to initiate noninvasive ventilation in children.
KEY TERMS
Sleep apnea
Hypoventilation
Continuous positive airway pressure
Oxygen
High-flow oxygen therapy
Noninvasive ventilation
Invasive ventilation
This chapter reviews interventions relevant to sleep-disordered breathing (SDB) in children. All these disorders can be diagnosed in the sleep laboratory and can be simply grouped as disorders of
upper airway obstruction (i.e., obstructive sleep apnea [OSA]),
restrictive lung function and/or poor muscle tone (i.e., hypoventilation), and
impaired respiratory drive (i.e., central sleep apnea [CSA], or hypoventilation).
Interventions that will be discussed include continuous positive airway pressure (CPAP), supplemental oxygen, and noninvasive and invasive ventilation. All forms of respiratory support therapy need to be adjusted to suit the patient requirements, and regular reviews and titrations are to be performed in the sleep laboratory to accommodate for growth and monitoring of disease progression.
DETERMINING WHICH RESPIRATORY SUPPORT TREATMENT TO USE
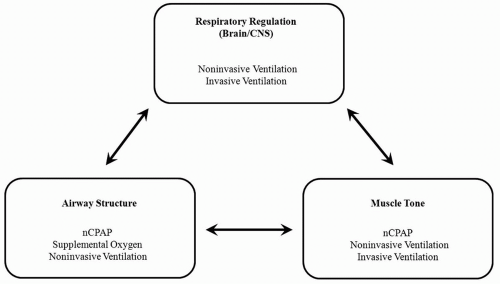
Optimal gas exchange is dependent upon three variables: (1) brain and central nervous system (CNS) function, (2) the structural design of the airways, and (3) muscle tone. If there is a fault with any one of these variables, then a child is at risk for developing SDB. On the basis of the child’s clinical history and physical examination, one can hypothesize the nature of the SDB the child will develop. Diagnostic polysomnography and blood gas testing allow clinicians to diagnose the problem and to determine the treatment to be initiated. Treatment recommendations may include CPAP, oxygen therapy, and/or ventilation (Fig. 65-1).
Central Respiratory Control
A number of conditions interfere with normal regulation of breathing by the brain and the CNS. Respiration is initiated in the pre-Bötzinger complex in the pons and medulla oblongata in the brainstem and further regulated by the carotid and aortic bodies. These areas submit electrical pulses to the diaphragm through the phrenic and thoracic nerves in the spinal column. Conditions associated with the spinal column or head, such as trauma, tumors, or malformations (e.g., an Arnold-Chiari malformation), can interfere with respiratory drive, respiratory rhythm, or respiratory regulation. The hypoxic response is primarily driven by the carotid bodies, whereas the response to carbon dioxide (CO2) is primarily driven by brainstem structures. These combined abnormalities result in susceptibility to CSA and/or hypoventilation and a need for the child to be treated with positive pressure or volume ventilation in order
to regulate the respiratory rate and/or tidal volume. If a child requires only nocturnal support, then noninvasive ventilation (NIV) is the optimal treatment. On the basis of modern technology, NIV is predominately positive pressure ventilation. If the individual requires assistance during wake and sleep and has a chronic condition, then invasive ventilation should be considered. Depending on the severity of the lack of respiratory drive, invasive ventilation can be pressure or volume ventilation.
to regulate the respiratory rate and/or tidal volume. If a child requires only nocturnal support, then noninvasive ventilation (NIV) is the optimal treatment. On the basis of modern technology, NIV is predominately positive pressure ventilation. If the individual requires assistance during wake and sleep and has a chronic condition, then invasive ventilation should be considered. Depending on the severity of the lack of respiratory drive, invasive ventilation can be pressure or volume ventilation.
Structural Compromise of the Airway
Structural abnormalities in the airway can interfere with optimal gas exchange by predisposing to narrowing or collapse of the airway. Obstruction to airflow can occur at any point along the airway from the nares to the alveoli. Examples of the physical obstruction in the upper airway include adenoid and/or tonsil hypertrophy, choanal atresia/stenosis, a cleft palate, or an enlarged tongue (macroglossia). These may be identified on physical examination or through more invasive testing procedures including nasendoscopy. Children at risk for upper airway obstruction may also be assessed using the Mallampati classification (1). Other causes of structural narrowing include micrognathia, a congenitally narrow airway, or childhood obesity whereby increased adipose tissue surrounds the muscular soft tissue of the upper airway. The primary example of a congenital floppy airway is laryngomalacia in infants, usually presenting soon after birth.
Surgical interventions can often alleviate these problems, but CPAP may be required for stabilization or long-term therapy when surgery is either contraindicated or not curative. The most easily corrected cause of obstruction is adenotonsillar enlargement, but other commonly performed surgical procedures include mandibular distraction and supraglottoplasty.
Gas exchange abnormalities can also be caused by structural abnormalities in the lower airway such as restrictive lung disease and/or chest wall abnormalities (i.e., kyphoscoliosis, cystic fibrosis, and bronchiectasis). It is important to note that morbidly obese children (particularly adolescents) may be at an increased risk of nocturnal hypoventilation because of the excessive adipose tissue deposited on the truck and abdomen restricting pulmonary function. If hypoventilation is present, treatment with NIV (with or without oxygen therapy) should be considered. It is common for children with lung disease (i.e., neonatal lung disease and cystic fibrosis) to be treated with supplemental oxygen, but in some cases, CPAP or NIV is also required because of associated conditions such as OSA or airway malacia.
Poor Muscle Tone
Gas exchange is also influenced by the effectiveness of respiratory muscle function. Many congenital myopathies are associated with progressive muscle weakness; the classic example is Duchenne muscular dystrophy. Mild loss of muscle tone can also affect the upper airway and predispose to upper airway obstruction, and treatment with CPAP therapy may be sufficient in these cases.
Congenital Heart Disease
The use of CPAP or NIV for patients with congenital heart disease (CHD) is often for the management of respiratory issues occurring secondary to the underlying cardiac condition. CHD can affect the respiratory system by causing obstructive and/or diffusion defects.
Direct anatomic compression of the airway can occur in association with cardiac abnormalities, secondary to the enlargement of cardiac structures or abnormal vascular structures (such as vascular rings). As an example, tetralogy of Fallot is associated with the narrowing of the pulmonary artery as well as ventricular septal defect, and leads to the deviation of the aorta to the right and thickening of the right ventricle. This in turn can lead to pulmonary artery dilation, bronchial or tracheal compression, and atelectasis, resulting in respiratory distress (2).
In terms of diffusion defects, ventricular or atrial septal defects can result in increased pulmonary blood flow because of left to right shunts (3). The increase in pulmonary blood flow can result in loss of elasticity in pulmonary vessels and increase the resistance in the pulmonary bed (4), thus impacting on gas exchange.
The use of CPAP or NIV should be aimed at relieving airway compression, increasing lung volume, equalizing transalveolar pressures (in addition to cardiac medications), and expanding surface area available for gas exchange to occur in the pulmonary bed (5).
CPAP THERAPY FOR CHILDREN
The most common reason for initiating CPAP in a child is to treat upper airway obstruction, predominantly OSA. But under what circumstances and how severe does the upper airway obstruction have to be before CPAP is warranted? There is no consensus on this matter, but from clinical experience, a number of factors can be evaluated to help with this decision. From diagnostic polysomnography, the main variables used include the following:
The obstructive apnea-hypopnea index (AHI)
Oxygen desaturation index and oxygen saturation nadir (SpO2)
Evidence of CO2 retention
Increased arousal frequency and fragmentation of sleep architecture
Increased work of breathing
OSA in children is diagnosed as an AHI greater than or equal to one event per hour (6, 7); however, CPAP is generally commenced only on children who have at least a moderate OSA or have a mild OSA with comorbidity(ies). A symptomatic child with mild OSA may be recommended CPAP if he or she has poor sleep quality, that is, an inability to fall asleep due to the obstruction, an elevated arousal index (>10 events per hour), and/or poor sleep architecture. Clinically, if the child has a high work of breathing, failure to thrive (8), poor development (9, 10), poor daytime performance and/or behavior, and/or respiratory distress, then CPAP may also be considered. Using CPAP alleviates the high work of breathing, thus conserving calorie consumption and allowing weight gain (11), and improves sleep quality and thus daytime function and behavior (12). Nasal steroids, body positioning, and surgical intervention (i.e., adenotonsillectomy) may be used without CPAP for milder cases of OSA, or used in conjunction with CPAP to help alleviate OSA in more severe cases (13, 14).
The most common correctable pathologies underlying OSA in children are adenoid and/or tonsil hypertrophy and obesity. The most common age for presentation with adenoid and tonsil hypertrophy as the cause of OSA is 2 to 5 years, and this subsequently declines with age. Conversely, the risk of OSA associated with obesity increases with age (15). Although most children with mild-to-moderate OSA respond to adenotonsillectomy (16), a proportion of children fail to respond to this surgery. Persistence of disease in a number of these children is reflected by the fact that a history of past adenotonsillectomy increases the risk of current OSA more than 2-fold, whether or not there is an underlying abnormality such as myelomeningocele (17, 18). Clinical practice has demonstrated that treatment with CPAP is a viable option for the majority of children who fail surgery. Treatment with CPAP is also increasingly recognized as effective for the management of perioperative airway obstruction (19, 20, 21, 22). Criteria for such intervention are becoming clearer as the predictors for perioperative problems are clarified (23, 24).
A number of common syndromes and conditions increase the risk and severity of OSA, and therefore increase the likelihood of requiring CPAP. These include Trisomy 21, achondroplasia, Pierre Robin sequence, Prader-Willi syndrome, cerebral palsy, and obesity. In these instances, although surgical intervention remains appropriate, it is likely to alleviate but not “cure” the OSA, and CPAP treatment may still be required for effective long-term management of the upper airway obstruction. Nonetheless, it is important to note that a proportion of children who present with OSA, particularly those presenting during infancy, show improvement as they get older (10). Our
experience suggests that if we can provide respiratory support during early development, many of these children are able to cease treatment. The caveat in this circumstance is that the presence of disease during childhood may be an indicator of small central and upper airways that will persist throughout life and into adulthood (25, 26). At the more severe end of the spectrum or where the diagnosis of upper airway obstruction is made in later childhood, the prognosis for cure appears to be poor.
experience suggests that if we can provide respiratory support during early development, many of these children are able to cease treatment. The caveat in this circumstance is that the presence of disease during childhood may be an indicator of small central and upper airways that will persist throughout life and into adulthood (25, 26). At the more severe end of the spectrum or where the diagnosis of upper airway obstruction is made in later childhood, the prognosis for cure appears to be poor.
Commencing CPAP Therapy
The data presented here regarding the commencement of CPAP in children are derived largely from clinical practice and experience. An intervention that can be specific for children includes behavioral strategies to assist with compliance. If hospital or home nursing resources are available, another strategy can be CPAP acclimatization in the presence of medical and nursing care. This provides family support, aids with adherence, and optimizes CPAP pressure. Otherwise, many of the other steps for commencing CPAP therapy follow adult practice (27). A few published studies address the issues surrounding CPAP initiation and adherence for children, and there has been limited formal evaluation of the acceptance by pediatric patients of different devices (28). However, what is known is that with proper training good adherence can be achieved in children (29, 30) and that it can be maintained with careful follow-up.
The strategies to commencing CPAP vary according to the age and development of the child. For infants (less than ˜6 months old), the therapy can generally begin immediately, with no need for prior behavioral programs. Instead, intervention strategies and education should be primarily geared toward the parents/primary caregiver because of their primary role in achieving adherence with therapy. The adaptation process for CPAP is approximately 3 to 7 days; however, in some cases, infants take to the therapy almost immediately.
For older children, success in implementing CPAP therapy is improved if it is commenced in a graduated manner. To maximize adherence, the children must first become adapted to the nasal mask. This takes approximately a week and is done in the home environment. Role-playing with the child (e.g., acting as an elephant, “Buzz Lightyear,” or an astronaut) assists with this. Allowing the child to wear the mask for 10 to 15 minutes when awake minimizes the “scare factor.” Once the mask is accepted, the child is admitted to hospital and a CPAP pressure is introduced. Others report successful introduction of CPAP in an immediate manner, with or without the addition of behavioral intervention programs (31).
In adolescents, in general the process is the same as for adult patients. Reports now suggest that adults utilizing CPAP also benefit from access to a multidisciplinary approach to aid adherence with therapy (32). Practice guidelines derived from evidence in the literature suggest that once a diagnostic study has demonstrated the presence of OSA, the patient is fitted with the mask interface, and the process is explained to the patient. A trial of CPAP can be undertaken with the patient awake and participating. An overnight sleep study for pressure titration can be undertaken almost immediately. Pressures are adjusted during the study and the patient is discharged the next day on the pressure setting deemed optimal on the basis of a single night of determination. Although this may be appropriate for the older adolescent, a hospital admission to commence therapy may still be appropriate for younger or developmentally delayed teenagers.
In all age categories, CPAP therapy is commenced on a low pressure (e.g., 5 cm H2O) to allow the child to get used to the sensation of the therapy and therefore maximize the likelihood of adherence. The first stage is to achieve overnight adherence with low-pressure therapy. A clinical review permits appropriate pressure changes to follow, for example, in the presence of ongoing snoring, pressures need to be increased. It is also important to note, depending on the age and complexity of a child’s condition, that commencing CPAP at home with the assistance of a community nurse may also help the child and parents adjust to using CPAP as part of their bedtime routine.
PRINCIPLES OF TITRATION
For disorders with upper airway obstruction, the goal of pressure support is to splint the airway open in order to relieve the obstruction and achieve normal ventilation. When performing polysomnography for CPAP pressure determination, the channels that should be closely monitored in order to achieve an optimum CPAP pressure are nasal airflow, respiratory effort, oxygenation (SpO2), and CO2 (33). Monitoring of CO2 during titration studies requires the use of transcutaneous (TcCO2) rather than end-tidal measurements (EtCO2). With the exception of children with lung or cardiac disease, the aim of a CPAP pressure determination study is to achieve a normal oxygen saturation (i.e., SpO2 ≥ 95%) and CO2 concentration (i.e., 35 ≤ TcCO2 ≤ 45 mm Hg). The pulmonologist and/or cardiologist should set discretionary guidelines for children with underlying/associated lung and/or cardiac disease.
Titrating CPAP during Polysomnography
Once the patient has adapted to the use of the pressure support, a full CPAP titration polysomnogram is undertaken in the sleep center. It is recommended that
titration studies follow the American Academy of Sleep Medicine (AASM) guidelines (34). In general, pressure titration studies commence with minimal positive pressure (e.g., 4 cm H2O) and do not exceed 15 cm H2O for children less than 12 years old. The priorities for pressure adjustment are to eliminate discrete obstructive respiratory events, eliminate signs of increased work of breathing, and eliminate signs of airflow limitation.
titration studies follow the American Academy of Sleep Medicine (AASM) guidelines (34). In general, pressure titration studies commence with minimal positive pressure (e.g., 4 cm H2O) and do not exceed 15 cm H2O for children less than 12 years old. The priorities for pressure adjustment are to eliminate discrete obstructive respiratory events, eliminate signs of increased work of breathing, and eliminate signs of airflow limitation.
Discrete obstructive events and labored breathing on the polysomnogram are seen as a loss of nasal airflow and increased respiratory effort with, or without, oxygen desaturations and/or arousals. The general practice is to increase the CPAP in 1 cm H2O increments if at least one obstructive apnea or two hypopneas are seen in children aged 12 or less or at least two apneas or three hypopneas are present in children greater than age 12. Each pressure change should be separated by at least 5 minutes of recording.
Once discrete obstructive events are eliminated, the next step is to put an end to nasal airflow limitation, residual snoring, and respiratory effort-related arousals (RERAs). The CPAP for children aged less than 12 years is to be increased if there are at least three RERAs or 1 minute of loud or unambiguous snoring. The CPAP is to be titrated higher for children 12 years or older who experience at least five RERAs or 3 minutes of loud or unambiguous snoring. CPAP is increased in approximately 1 cm H2O increments until this is achieved.
Once optimal pressure is achieved, there should be no snoring or stridor, and there should be normal nasal airflow and respiratory effort, stable oxygenation without intermittent desaturations, and normal CO2. Allowing 30 to 60 minutes before additional incremental increases in CPAP will provide sufficient time for the patient to blow off any excess CO2 and allow adequate sleep for analysis by the assessing physician. To allow the sleep physician to assess and confirm the optimal pressure, an ideal study includes pressure adjustments to a level slightly above the optimal CPAP. Acute signs that the CPAP has been increased too high include increased frequency of arousals, recurrence of the use of accessory muscles, fall in baseline oxygenation and CO2 retention, and the occurrence of central (rather than obstructive) respiratory events (9). AASM standards indicate that an optimal CPAP is achieved when the respiratory disturbance index (RDI) is less than five events per hour for at least 15 minutes, SpO2 is above 90%, and a rapid eye movement (REM) period in the supine position is recorded (34). The titration is considered good when the RDI remains greater than 10 events per hour and SpO2 is above 90%. The titration may be adequate if the RDI has been reduced by 75% from diagnosis or titration was not assessed during supine REM sleep. AASM standards indicate that a titration is unacceptable when any of the above-mentioned criteria remain unmet.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree