Fig. 6.1
(a) Illustration of microcatheter placement into an MCA clot. (b) Angiogram demonstrating acute occlusion of the M1 segment of the MCA (arrow). (c) Recanalized MCA after 600,000 U IA UK
Early attempts at IA thrombolysis for carotid artery territory occlusions include the reports of Sussman et al. [20], Atkin et al. [21] and Labauge et al. [22]. Zeumer [23] is often credited with ushering in the modern era of IA stroke thrombolysis in 1983 with a series of case reports describing IA thrombolysis for internal carotid artery occlusion using either urokinase (UK) or streptokinase (SK).
In 1988, del Zoppo et al. [24] reported 20 patients treated with either IA SK or UK. Complete recanalization occurred in 15 cases (75 %) and 10 patients (50 %) had improvement of neurological symptoms. There were 3 deaths (15 %) and 3 patients (15 %) with embolic strokes in his series.
Also in 1988, Mori et al. [25] reported a series of 22 patients treated for acute occlusion of the MCA with IA UK, in doses ranging between 0.8 and 1.32 million units. Recanalization occurred in 10 cases (45 %), of which 4 were complete and 6 had residual stenosis. There was symptomatic improvement in 8 of the 10 (80 %) cases with recanalization. In addition, there was a significant correlation between recanalization and improved clinical outcome in his series.
Theron et al. [26] reported 12 patients with carotid territory occlusions treated with local IA SK or UK. Most patients were treated within 10 h of stroke onset, although in one case symptoms had recurred over 5 weeks. Theron et al. speculated that occlusions involving the lenticulostriate vessels carry the highest risk of hemorrhage since the 2 symptomatic brain hemorrhages in this series both occurred among the 5 patients with occlusions at this level.
Zeumer et al. [27] reported their experience with local IA UK (750,000 IU) or rt-PA (20 mg) given over 2 h in 31 patients with acute carotid territory and 28 patients with acute vertebrobasilar occlusion. All patients received a bolus injection of 5,000 U heparin, followed by a 1,000 U/h intravenous heparin infusion during the procedure. In carotid territory patients, treatment had to be finished by the 6th hour after stroke onset. Assuming a very bad prognosis, no time limit was placed on vertebrobasilar cases and the average delay to treatment was 8 h. In the carotid territory, recanalization was achieved in 94 % of patients (complete 38 %). Five types of carotid territory occlusions were identified: (1) carotid siphon C1/2 segment only; (2) MCA M1; (3) MCA M1 plus M2; (4) MCA M2 or M3; (5) Multiple occlusions or multiple emboli beyond M1 and A1. Optimal recanalization and clinical results were obtained only in type 1 and type 4 occlusions. The neurological deficit was minimal or mild in 32 %. In the vertebrobasilar territory, a 100 % recanalization rate was achieved (complete 75 %). The mortality rate was 65 %; 7 patients (25 %) had a minimal or mild deficit. There were no brain hemorrhages with clinical neurological deterioration, and no apparent difference in efficacy between IA UK and rt-PA.
Higashida et al. [28] in 1994 also reported their results in 27 cases who were treated for an acute arterial occlusion in 45 vascular territories. Clinically there was neurological improvement in 18 (66.7 %) cases. Complications directly related to therapy included symptomatic intracranial hemorrhage in 3 cases (11.1 %). In 8 (29.6 %) patients, there was no evidence of clinical improvement and in long term follow up there were 9 (33.3 %) patient deaths.
In an attempt to speed the time to recanalization, Freitag et al. [29] compared 40 patients with carotid territory stroke treated with IA UK or IA rt-PA, to 15 patients treated with up to 30 mg IA rt-PA plus lys-plasminogen (PG). Only l patient (2 %) experienced brain hemorrhage with clinical neurological deterioration. 40 % of the UK-rt-PA patients had a Barthel index score >90 at 3 months compared to 60 % in the lys-PG/rt-PA group. For vertebrobasilar patients, long term survival was 50 % in the UK—rt-PA group (n = 20), and 58 % in the lys-PG/rt-PA group (n = 12).
Matsumoto and Satoh [30] have studied IA thrombolysis in 93 patients (1995). This series is atypical in that a 24 h entry window was used. 57 patients received regional IA UK with a maximal does of 1,200,000 IU. 19 patients received local IA UK, and 18 patients received local IA rt-PA. Among the 36 patients with ICA occlusions, none completely recanalized with regional UK (n = 21), while 33 % recanalized with local IA UK (3 of 8) or IA rt-PA (2 of 7). Outcome was said to be good or excellent in 8 patients (22 %); the mortality rate was 44 % (16 of 36). 41 patients had MCA occlusions. The MCA complete recanalization rates were: regional UK, 62 % (13 of 21); local UK, 64 % (7 of 11); local rt-PA, 78 % (7 of 9). Clinical outcome was good or excellent in 37 % of all MCA patients, and 50 % for patients treated with local thrombolysis. Overall MCA mortality was 22 % (9 of 41). The parenchymal hematoma rate for ICA occlusion was 6 % (2 of 36), and for MCA occlusion 10 % (4 of 41). 14 of 16 patients with basilar artery occlusion received regional UK (2 local rt-PA). Forty-four percent of basilar artery cases had a good or excellent outcome, while 31 % died. There was 1 parenchymal hematoma (6 %) in the basilar artery group. Gotoh and Ogata [31] reported a 93 % recanalization rate in 14 patients (12 MCA) treated with local IA UK starting at the distal clot interface. There were no complications.
Sasaki et al. [32] retrospectively reviewed 95 cases of thrombolysis for acute stroke at their institution between 1983 and 1992 to determine whether the location of infusion affected the results. Forty-four patients were given either IA UK or rt-PA to the local area of vascular occlusion, while 18 patients were given intra-carotid infusion of UK. Only 2 of 18 patients receiving intra-carotid UK for M1 occlusion experienced even partial recanalization; the other 16 patients demonstrated no change. In the local fibrinolytic group, complete recanalization occurred in 52 % of patients and partial recanalization was achieved in another 32 % of patients. The highest rates of recanalization were seen in M1 and basilar artery occlusions, while the lowest rate of recanalization was seen in patients with ICA siphon occlusions. The reduction in infarction size and outcome were good in patients with complete recanalization. No differences were observed between IA UK and IA rt-PA in achieving recanalization. Patients were given an intra-procedural infusion of up to 5,000 U of heparin which was stopped at the conclusion of the procedure. Hemorrhagic infarction was seen in 22 % of patients at 24 h post-IA thrombolysis, although the number of symptomatic hemorrhages was not identified.
Gönner et al. [33] retrospectively analyzed a series of 43 consecutive patients treated with IA thrombolysis and found that there was a statistically significant improvement in the success of recanalization if therapy was initiated within 4 h of stroke onset compared to patients treated after 4 h. Lansberg and colleagues [34] reported a 73 year old patient who received IA rt-PA into three occluded left hemispheric vessels. On DWI there was no abnormality in the region of the vessel successfully recanalized under 3 h from onset, but there were DWI abnormalities in the region of the artery recanalized at 3.5 h and in the territory of the vessel that failed to reopen.
Qureshi et al. [35] studied 8 consecutive patients given IA rt-PA to determine the most efficacious dosing regimen. Interval from presentation to treatment ranged from 1 to 8 h. Patients were given escalating doses of local IA rt-PA beginning with 10 mg and incrementally grading reperfusion using a modified TIMI scale to a total possible dose of 40 mg. The infusion was stopped if recanalization was achieved. Increasing mean perfusion grades were seen with higher doses of rt-PA. 4 of the 8 patients (50 %) experienced neurologic improvement after IA rt-PA. No anticoagulation was given as part of the procedure. 2 of the 8 (25 %) patients exhibited an asymptomatic hemorrhage at 24 h post-infusion.
Qureshi et al. [36] prospectively treated 16 consecutive patients with an acute stroke with an NIHSS 10–26 between 2 and 9 h from the time of onset. Patients were given up to 8 U of IA reteplase. 7 of 16 patients received adjunctive angioplasty of the occluded vessel. Modified TIMI grade 3 or 4 was achieved in 88 % of patients and modified TIMI 2 in 1 more patient. Only 1 patient did not achieve at least partial recanalization. Forty-four percent experienced a 4-point or better improvement in the NIHSS. 4 of the 16 (25 %) patients experienced an intracerebral hemorrhage seen on CT at 24 h post-thrombolysis. Only 1 of the 16 (6 %) patients had a symptomatic hemorrhage. The overall mortality was 56 % largely owing to massive ischemic strokes.
Embolic occlusions of the intracranial vasculature may result as a complication of neuroendovascular procedures. Hähnel et al. [37] retrospectively reported 9 of 723 patients who underwent a neuroendovascular procedure which was complicated by thromboembolism. Time from detection of embolic occlusion until the start of IA rt-PA infusion was between 10 and 90 min. Despite successful recanalization in 4 of the 9 patients, and the relatively short duration from onset to drug infusion, all patients suffered infarcts and were at least moderately disabled at 3 months.
The relationship between recanalization, rt-PA dose and occlusion type has been examined by Eckert et al. [38]. One hundred and thirty-seven (137) patients with angiographic occlusion of the anterior circulation within 6 h of symptom onset received either urokinase, low (10–20 mg) or high dose (40–90 mg) rt-PA, or rt-PA plus lys-plasminogen. 57 % of patients with good neurologic outcome (Barthel index score >90) were recanalized, whereas only 10 % of non-recanalized patients had a good outcome. Occlusion type had a significant impact on neurologic outcome. Patients with proximal M1 occlusions had higher rates of recanalization (70 %) and a higher rate of moderate to good outcomes (80 % with Barthel index score >50) at 3 months. Recanalization rates were slightly better with high dose rt-PA vs. lose dose rt-PA (50 % vs. 32 %, respectively), but were highest with the combination of rt-PA and lys-plasminogen (83 %) with similar rates of hemorrhage. Among 35 patients with occlusion of the carotid T, a poor neurologic outcome (Barthel index score <50) was seen in 49 % of patients, and the mortality rate was 43 % of patients at 3 months. In addition, Arnold et al. [39] demonstrated that the sufficiency of leptomeningeal collaterals is a predictor of favorable outcome in patients with carotid T occlusions.
Selected Case Series IA Thrombolyis: Vertebrobasilar Territory (Fig. 6.2)
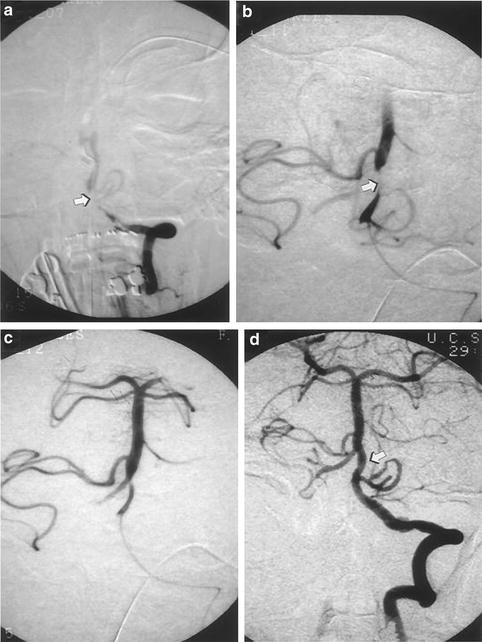
Fig. 6.2
(a) Angiogram demonstrating acute thrombosis of the junction of the distal left vertebral and basilar artery (arrow). (b) Microcatheter placement within proximal basilar artery. Thrombus partially recanalized after 200,000 U IA UK (arrow). (c) Further recanalization of basilar artery thrombus. (d) Final basilar artery recanalization (arrow) after 600,000 U IA urokinase
The natural history of basilar occlusion is generally poor with mortality ranging from 83 to 91 % [40]. Because of this poor natural history, IA thrombolysis has been preferred in patients with acute basilar artery occlusion. Approximately 278 cases have been reported with an overall basilar artery recanalization rate of 60 % [40]. Basilar artery occlusions are usually due to atherothrombosis. There is a high incidence of residual stenosis after basilar artery recanalization which often requires adjuvant therapies including angioplasty and/or stenting, antithrombotic, and antiplatelet agents.
In a compilation of reported cases of vertebrobasilar IA thrombolysis, the mortality in patients failing recanalization was 90 % compared to 31 % mortality in patients achieving at least partial reperfusion [41]. Good outcomes are strongly associated with recanalization after thrombolytic therapy. Hacke et al. [42] described 65 consecutive patients with vertebrobasilar occlusion treated either with local IA UK, or IA SK plus heparin (n = 43), or conventional antiplatelet/anticoagulation therapy (n = 22). The recanalization rate among thrombolysis cases was 44 % (19 of 43). All patients without recanalization died, while 14 of 19 with recanalization survived, 10 with a favorable outcome. The mortality rate with conventional therapy was 86 % compared to 67 % with thrombolysis. The rate of brain hemorrhage with clinical neurological deterioration was 7 % in thrombolysis patients. Schumacher et al. [43] reported 29 patients with vertebrobasilar occlusion of <6 h duration treated with up to 1,500,000 IU local UK plus IV heparin. Recanalization was achieved in 66 %. There was no or minimal deficit in 45 %. The mortality rate was 45 %. Eckert et al. [13] performed IA thrombolysis using 30 mg of rt-PA given over 2 h in combination with 12 h of IV abciximab therapy (0.25 mg/kg bolus followed by 0.125 mcg/kg/min infusion) in 3 patients. Recanalization occurred in 2 of 3 patients, both of which achieved clinical independence at 3 months post-thrombolysis.
Recanalization rates depend upon the location of the vertebrobasilar occlusion. Distal basilar occlusions have higher recanalization rates than proximal occlusions. Emboli often lodge in the distal basilar artery and are easier to lyse than atherosclerosis related thrombi, the usual cause of proximal basilar occlusions [44]. Short segment occlusions are easier to lyse than longer segment occlusions [45]. Younger patients have higher recanalization rates [46], probably due to the increased incidence of embolic occlusions seen in this age group.
The timing of IA vertebrobasilar thrombolysis is often a difficult decision. The presence of coma or tetraparesis for several hours portends a poor prognosis, despite recanalization [42, 44, 46]. Such symptoms do not preclude survival, however, and recovery has been documented after successful recanalization in such patients [44, 45, 47].
The time window for thrombolysis may be longer in the vertebrobasilar circulation. Many series have included patients up 24 h [42], 48 h [48], and even 72 h [45], after symptom onset in patients with stuttering courses. An association between time to treatment and outcome has been suggested [49] but many series do not support this [45, 50, 51]. In fact, in some studies the time to treatment was actually longer in patients who survived or had good outcomes [45, 50]. A longer time window may be due to a higher ischemic tolerance or improved collateralization in the posterior circulation. Cross and colleagues [45], reporting on 20 patients with basilar artery thrombosis who received IA thrombolysis, found that better collateral blood flow was correlated with improved responses to thrombolysis and with longer tolerance of ischemia. Patients with proximal basilar artery thrombosis did not seem to have the same benefit.
Patients with vertebrobasilar ischemia often have chronic atherosclerotic disease which allows collaterals to develop over time. As hypothesized by Cross et al. [45] there may be two distinct populations of patients with vertebrobasilar occlusion. Patients with a progressive stuttering course may have better collateral circulation and have better outcomes despite later treatment than patients with the sudden onset of severe deficits due to poor collaterals who may be brought to treatment earlier.
While some authors believe that patients with brainstem infarction on CT are not candidates for thrombolytic therapy [42, 44], others have found no correlation with neurologic outcome [45, 50]. In two separate series, none of the patients who had CT evidence for brainstem ischemia developed a hemorrhage. However, because of the experience in the anterior circulation, caution should be used when considering thrombolysis in patients with early infarct signs.
The Prolyse in Acute Cerebral Thromboembolism Trials (PROACT I and PROACT II)
The only randomized, controlled, multicenter trials of IA thrombolysis in acute ischemic stroke are PROACT I [10] and PROACT II [1]. In PROACT I, the safety and recanalization efficacy of 6 mg recombinant pro-urokinase (r-proUK) was examined in 40 patients with acute ischemic stroke of less than 6 h duration due to occlusion of the MCA. The control group received IA saline. The recanalization rate was 57.7 % in the r-proUK group and only 14.3 % in the placebo group. Two doses of heparin were used in PROACT I. In the high heparin group (5,000 U bolus followed by 1,000 U per hour infusion) the recanalization rate was 80 %, but the symptomatic ICH rate was 27 %. In the low heparin group, the recanalization rate was 47 % but the ICH rate was decreased to 6 %. Although not a clinical efficacy trial, there appeared to be a 10–12 % increase in excellent outcomes in the IA r-proUK group.
The follow-up clinical efficacy trial, PROACT II [1], was launched in February, 1996 and completed in August, 1998. The results were first reported in February, 1999. PROACT II used an open design with blinded neurological follow-up. The vast majority of patients in PROACT II were IV rt-PA ineligible due to arrival beyond 3 h. Also, mechanical clot disruption was prohibited. Patients were screened with conventional angiography for occlusion of the MCA and had to have a NIHSS score between 4 and 30. The patients in PROACT II had a very high baseline stroke severity score with a median NIHSS of 17. Patients with early signs of an infarct in greater than one third of the MCA territory (ECASS criteria) on the baseline CT scan were excluded from the study. One hundred and eighty (180) patients were then randomized to receive either 9 mg of IA r-proUK plus low dose IV heparin or low dose IV heparin alone. The primary outcome measure was the percent of patients who achieved a modified Rankin score of ≤2 at 90 days, which signified slight or no neurological disability. Secondary measures included the percentage of patients who had a NIHSS ≤1 at 90 days, angiographic recanalization, symptomatic intracerebral hemorrhage, and mortality. The median time from onset of symptoms to initiation of IA thrombolysis was 5.3 h.
In the r-proUK treated group there was a 15 % absolute benefit in the number of patients who achieved a modified Rankin score of ≤2 at 90 days (P = 0.043). Therefore, on average, 7 patients with MCA occlusion would require IA r-proUK for one to benefit. The benefit was most noticeable in patients with a baseline NIHSS between 11 and 20. Recanalization rates were 66 % at 2 h for the treatment group and 18 % for the placebo group (p<0.001). Symptomatic brain hemorrhage occurred in 10 % of the r-proUK group and 2 % of the control group. In PROACT II, as in the NINDS trial, despite the higher early symptomatic brain hemorrhage rate, patients overall benefited from the therapy and there was no excess mortality (r-proUK 24 %, control 27 %).
Brain Hemorrhage and IA Thrombolysis
Aggregate data indicates an 8.3 % risk of symptomatic brain hemorrhage with IA thrombolysis in the carotid territory and a 6.5 % risk in the vertebrobasilar territory [41]. There is no evidence that the rate of symptomatic brain hemorrhage is lower with IA thrombolysis than with IV thrombolysis, but direct comparisons are difficult. In an uncontrolled series, Gönner et al. [33] reported a 4.7 % rate of symptomatic brain hemorrhage in 42 patients treated with IA thrombolysis. This series differed from PROACT II in that only 26 out of the 42 patients received heparin; the remainder received aspirin. The higher rate of intracranial hemorrhage with neurological deterioration with IA rpro-UK in PROACT II (10.2 %) compared to IV rt-PA in NINDS (6.4 %) [2], ATLANTIS (7.2 %) [52] and ECASS II (8.8 %) [53] must be understood within the context of the greater baseline stroke severity, longer time to treatment, and 66 % MCA recanalization rate in PROACT II. The median baseline NIHSS score in ATLANTIS and ECASS II was 11, in NINDS 14 and in PROACT II 17. Greater baseline stroke severity was first associated with increased intracranial hemorrhage risk in NINDS and ECASS I. All symptomatic intracranial hemorrhages in PROACT II occurred in patients with a baseline NIHSS score ≥11, and in NINDS the rate of symptomatic brain hemorrhage in patients with a NIHSS >20 was 18 %.
Although brain hemorrhage complicating thrombolysis for acute stroke likely reflects reperfusion of necrotic tissue, several IV thrombolysis series have found no direct relationship between recanalization and hemorrhage risk [54, 55]. Kidwell et al. [56] retrospectively reviewed 89 patients treated with IA thrombolysis at their center and found that the leading predictors of any hemorrhage in this population were the baseline NIHSS score, platelet count, glucose level and a longer time to recanalization. Also, this uncontrolled series from Kidwell et al. showed a strong trend toward a higher rate of hemorrhagic transformation in patients without recanalization vs. with recanalization (54 % vs. 33 %, respectively; p = 0.1) The amount of ischemic damage is a key factor in the development of brain hemorrhage after thrombolysis induced recanalization. Major early CT changes and severity of the initial neurological deficit, both indicators of the extent of ischemic damage, are some of the best predictors for the risk of hemorrhagic transformation [55, 57].
Several other factors have been associated with hemorrhage after thrombolysis for both stroke and myocardial infarction, including thrombolytic dose [58], blood pressure [55, 59, 60], advanced age, prior head injury [61] and blood glucose >200 mg/dl. Levels of blood matrix metalloproteinase-9 (MMP-9) in patients prior to thrombolytic therapy has been shown to be a risk factor for hemorrhagic transformation [62], although a specific genotypic relationship could not be elucidated [63]. Other studies to identify patients at risk have used advanced T2-weighted MRI sequences such as gradient echo planar imaging to identify patients with a history of microbleeds which may predispose to a higher rate of hemorrhagic transformation during thrombolysis [64]. Adjunctive antithrombotic therapy may also play a role during IA thrombolysis. Age was the most important risk factor in one of the largest series of thrombolysis-related intracranial hemorrhage [61]. A relationship between advanced age and hemorrhage was demonstrated in the NINDS [3] and ECASS trials [53, 57]. Although there is no strict age cutoff for administering thrombolytics for stroke, physicians need to take age into account, especially in patients over age 75 when determining the risk of angiography and IA thrombolysis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





