Location (sinus)
Typical feeding arteries
Venous outlet
Typical symptoms
Transverse/sigmoid (50 %)
ICA, MMA, APA, PA, OA, VA
Transverse/sigmoid sinus
Pulsatile tinnitus, bruit, headaches
Cavernous sinus (10–16 %)
ICA, IMA, MMA, APA
Cavernous sinus, ophthalmic vein, inferior petrosal sinus
Chemosis, proptosis, bruit, vision loss
Tentorial incisura (8–12 %)
ICA, MMA, APA, OA, VA
Straight sinus, deep cerebral veins
ICH, tinnitus, headache
Convexity (SSS) (8 %)
OphA, MMA, OA, VA
Superior sagittal sinus
Headache, dementia, seizures
Anterior cranial fossa (5 %)
OphA, IMA, MMA
Olfactory vein, frontal veins
ICH, headache
Foramen magnum (5 %)
ICA, MMA APA, OA, VA
Clival plexus
Myelopathy, ICH, tinnitus
Imaging of DAVF
Non-contrast Computed Tomography
As with most vascular lesions, DAVFs are not typically visible by non-contrasted computed tomography (CT). In patients with a high clinical suspicion, the following features might be noted:
The presence of intracranial hemorrhage, sometimes associated subdural hematoma
Ventriculomegaly secondary to communicating hydrocephalus
Hyperdensity of a dural sinus suggesting thrombosis
CT Angiography
Due to the high-flow nature of DAVF and the fact that they are commonly located near bone, the location of the fistulous connection is not generally visualized by CT angiography. Nevertheless, CT angiography can often identify some of the associated findings of the fistula including:
Venous varices
Dilation and/or occlusion of a venous sinus
Dilation of the ophthalmic vein
Abnormally enlarged subpial vessels
Magnetic Resonance Imaging
DAVFs are typically not well seen on magnetic resonance imaging (MRI). As with CT/CT angiography, MRI may identify dilated veins resulting from venous hypertension. In some cases, venous hypertension may cause focal areas of hyperintensity on T2 and FLAIR sequences. Dilated cortical veins may appear as flow voids within the cortical sulci but lack a true nidus within the brain parenchyma as would be found in a cortical AVM.
MR Angiography
Time-of-flight MR angiography, unlike CT angiography, is sensitive to the direction of blood flow. In some cases this will allow visualization of flow reversal in a dural sinus. As with other noninvasive imaging techniques, however, MR angiography often does not provide enough detail to aid in treatment planning.
Catheter Angiography
Catheter Angiography is the gold standard for the identification and staging of DAVF. In most cases, selective, bilateral injections the ECAs and ICAs as well as the VA should be performed to identify all of the arterial feeders. Often the fistula will have multiple feeders that supply a relatively short segment of the involved dural sinus. Retrograde flow from the arterialized sinus into the cortical veins (cortical venous reflux) is evidence of a hemodynamically significant change in local parenchymal venous drainage and has been associated with a more malignant natural history, including a higher rate of intracranial hemorrhage. The goals of catheter angiography include:
Locate arterial feeders.
Identify the fistulous portion of the involved sinus.
Look for evidence of venous sinus stenosis or reflux.
Evaluate alternative venous drainage pathways.
Look for evidence of cortical venous reflux.
Angiographic Classification of DAVF
Various classification methods have been adopted that attempt to explain the significance of the angiographic anatomy, namely, the pattern of venous drainage. The commonly used classifications are the Borden and Cognard classifications.
The Borden classification system is used to classify DAVF according to the patency of the affected dural sinus and the presence or absence of cortical venous reflux [4]. This classification system was initially proposed as a guide to help describe DAVF according to anatomical and physiological parameters and by doing so help in selecting the best surgical and/or endovascular approach. Later case series [5] confirmed that the Borden grade also correlates with the risk of intracranial hemorrhage or nonvisual neurological deficit. A summary of this classification system is presented in Table 13.2.
Borden type | Description | % of patients presenting with ICH or nonvisual neurological deficit |
|---|---|---|
I | Flow into the affected sinus is antegrade with no evidence of cortical venous reflux | 2 % |
II | Flow in the affected sinus is antegrade, but there is evidence of cortical venous reflux | 39 % |
III | There is no antegrade flow through the affected sinus distal to the fistula. All venous outflow from the fistula is via subarachnoid veins | 79 % |
Subtype A | Fistula has one arterial feeder | – |
Subtype B | Fistula has more than one arterial feeder | – |
The Cognard classification system is more detailed (Table 13.3) and elaborates on the direction of flow, whether normal (antegrade) or retrograde, and the presence or absence of cortical venous retrograde drainage. In addition, spinal perimedullary venous drainage is recognized [6].
Cognard type | Pattern of venous drainage |
|---|---|
I | Venous drainage into a sinus, normal antegrade flow |
II | Venous drainage into a sinus, with insufficient antegrade flow and reflux: |
IIa | – Retrograde venous drainage into a sinus only |
IIb | – Retrograde venous drainage into a cortical vein only |
IIa+b | – Retrograde venous drainage into a sinus and cortical veins |
III | Venous drainage into a cortical vein without ectasia |
IV | Venous drainage directly into a cortical vein with venous ectasia larger than 5 mm in diameter and three times larger than the diameter of the draining vein |
V | Venous drainage into spinal perimedullary veins |
Differential Diagnosis
Pial AVFs (PAVFs) arise from the arterial supply to the pia or cortex, and the abnormality does not lie with the dural leaflets. These rare lesions disproportionately affect pediatric patients and young adults. They present with similar symptoms and signs to DVAF, but intraparenchymal hemorrhage may be more common than subdural hemorrhages. The venous drainage is also cortical, and there may be an associate varix. Treatment modalities are similar to DAVF, but the more simple angioarchitecture makes transarterial embolization or surgical disconnection more feasible [7].
Arteriovenous malformations (AVMs) are distinguished from DAVF by the cobweb of fragile connecting blood vessels between the arterial input and the venous output present in the AVM. This network is known as the nidus (see Chap. 12).
Illustrative Case 1
A 27-year-old woman presented with sudden-onset headache and mild confusion. She was found to have a left, frontal lobe, intraparenchymal hemorrhage on non-contrast head CT (Fig. 13.1a). Diagnostic angiography showed a PAVF arising from the frontopolar branch of the left anterior cerebral artery (ACA) and draining into a cortical vein (Fig. 13.1b).
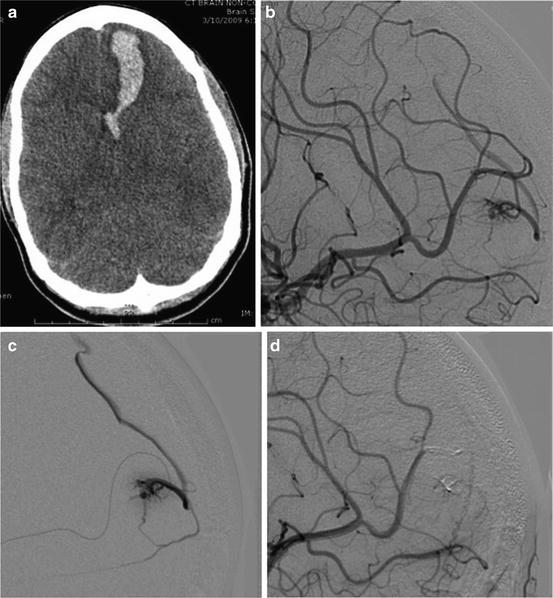

Fig. 13.1
Non-contrast head CT showing a left frontal lobe hemorrhage (a). Lateral projection angiogram showing a PAVF between the left frontopolar branch and a cortical vein (b), confirmed on microcatheter angiogram (c). Complete occlusion is shown after embolization with Onyx 18 (d)
A 6F 10 cm Pinnacle introducer sheath (Terumo; Somerset, NJ) was inserted within the right femoral artery. A 6F MPC Envoy (Codman Neurovascular; Raynham, NJ) along with a 0.035 in. Glidewire (Terumo; Somerset, NJ) was then used to select the distal left cervical internal carotid artery. A Marathon microcatheter and Mirage microwire were then navigated to the site of the fistula, and a microcatheter angiogram was performed (Fig. 13.1c). Onyx 18 liquid embolic was then used to occlude the PAVF (Fig. 13.1d).
The Natural History of DAVF
The natural history of a DAVF is primarily determined by its effect on local drainage patterns. Lesions that do not result in hemodynamically significant venous hypertension may produce only a bruit or pulsatile tinnitus. As the flow through the lesion increases, progressive venous hypertension develops. Depending on the location, this may lead to hydrocephalus, seizures, ocular edema, or myelopathy. High-flow lesions may eventually lead to cortical venous reflux causing the formation of venous varices and even intracranial hemorrhage.
The natural history of a DAVF is highly correlated with its Borden classification regardless of its location. Davies et al. [8] showed that both treated and untreated Borden grade I lesions had a benign clinical course. In 133 patient-years of follow-up, the following were observed:
Most patient’s symptoms improved with time with treatment (86 %) or without (81 %).
The rate of ICH, neurological deficit, or death was low (<2 %) in both treated and untreated patients.
Two patients out of 22 experienced a complication from embolization including a pulmonary embolus and an asymptomatic pericallosal artery embolus, but neither suffered permanent injury.
In contrast to Borden grade I lesions, Davies demonstrated that Borden grade II and III lesions are associated with a more malignant course [9].
29 % of untreated patients died during the 249 lesion-month follow-up period.
Untreated patients had a 19.2 % risk of ICH, a 10.9 % risk of neurological deficit, and a 19.3 % risk of death per year.
Endovascular cure was effective in avoiding symptoms, but patients with angiographic evidence of cortical venous reflux after treatment still had an aggressive clinical course.
Surgical resection of the fistula was associated with a high rate of complications (33 %). Surgical disconnection of the sinus alone was safer (no complications in 16 patients) and effective.
Medical Decision-Making
Based on this data, the Borden classification can be used to help choose between observation and treatment.
Incidental Borden grade I lesions can be safely observed. Endovascular embolization of these lesions is safe and may be considered for palliative treatment in symptomatic patients.
Borden grade II and III lesions have an aggressive clinical course and should be treated. Endovascular treatment is effective only if cortical venous reflux is eliminated. Surgical disconnection of the sinus was another safe and effective alternative.
Treatment Strategies for DAVF
The treatment strategies for DAVF include transarterial embolization, transvenous embolization, dural sinus recanalization, surgical sinus skeletonization, and radiation. Often, more than one technique will be needed to treat a complex lesion.
As with any arteriovenous shunt, the most effective and durable treatment consists of occlusion of the venous recipient of the fistula [10], whether this is achieved by endovascular treatment or surgery. A proximal arterial occlusion is usually not sufficient, despite angiographic non-opacification of the fistula, as the fistulous site will still be active. With dural collaterals, the fistula in time will recruit other dural supply and will recur. Conversely, it is equally important to ensure that sufficient venous drainage is preserved to avoid venous infarction or intracerebral hemorrhage.
1. Transverse-Sigmoid Sinus DAVF
The transverse-sigmoid sinus is the most common location for cranial DAVF. These lesions are often Borden type 1 and therefore have a benign natural history. Treatment is only to alleviate symptoms such as pulsatile tinnitus. Lesions with high-risk features such as cortical venous reflux, however, should be treated.
Selection of the treatment strategy involves weighing several different factors including the significance of the patient’s symptoms and their demand for treatment, as well as careful analysis of the angiogram. The angiogram may show that the affected sinus does not participate in normal venous drainage. In such cases venous occlusion has been shown to be effective and safe. Compartments of a sinus may be involved by the fistula, while others are free so that selective occlusion can be achieved while keeping the normal venous pathway patent [11]. When the targeted sinus is participating in normal cerebral drainage, cure by endovascular means is difficult, because in such cases the patent dural sinus is still a normal pathway for venous drainage, and its occlusion may risk a venous infarction. Palliative arterial embolization may thus be used for symptomatic relief. Arterial supply to fistulae of this region typically includes the petrous and petrosquamosal divisions of the middle meningeal artery, lateral division of the meningohypophyseal trunk off the ICA, transosseous branches of the posterior auricular artery, ascending pharyngeal artery, transosseous branches of the occipital artery, posterior meningeal arteries, and artery of the falx cerebelli.
In a large series of DAVFs of the transverse and sigmoid sinuses in 150 patients [12], the occlusion rate of transarterial embolization alone was 30 %, and multiple procedures were often required. Ideally, transarterial embolization with permanent liquid material (as Onyx) that closes only the AV shunts within the wall of the sinus is desirable. Some have proposed the use of transvenous balloon protection within the dural sinus to facilitate closure of the DAVF with preservation of the sinus [13]. Treatment of a transverse sinus DAVF by sinus recanalization by angioplasty and stenting together with transarterial embolization has also been reported [14].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








