11 | Intramedullary and Extramedullary Spinal Cord Tumors |
 | Case Presentation |
Case 1
A 34-year-old male experienced episodes of stumbling and falling 2 months prior to presentation. The patient first developed left leg numbness and weakness that progressed to his right leg. The sensory disturbance and weakness manifested as gait difficulties. He denied bowel, bladder, or sexual dysfunction. The patient’s past medical history and family history were noncontributory.
On physical examination, the patient had normal upper extremity strength and reflexes. He had normal pinprick and joint position sense bilaterally in his upper extremities. His right lower extremity exhibited 5/5 strength during hip flexion, 4/5 strength during knee extension, and 2/5 strength when dorsiflexing and plantar flexing the ankle. His left lower extremity revealed 4/5 strength during dorsiflexion and plantar flexing the ankle and normal strength proximally. Sensory loss to pinprick and temperature was observed bilaterally below the chest but joint position sense was intact. His lower extremity reflexes were normal and his toes were downgoing after Babinski reflex testing. He had normal cutaneous perianal sensation and rectal tone.
During his evaluation the patient had a brain, as well as cervical, thoracic, and lumbar spine magnetic resonance imaging (MRI) with and without gadolinium. The scan demonstrated an intradural-extramedullary mass with significant cord compression at the cervical-thoracic junction (Fig. 11–1). The mass enhanced homogeneously and originated dorsal to the spinal cord. A small enhancing dural tail was appreciated.
The patient was taken to the operating room and underwent an osteoplastic laminectomy and resection of the lesion. Pathology demonstrated that the lesion was consistent with a meningioma. Gross total resection was achieved. The dural base of the tumor was excised. Duraplasty was performed with dural substitute. Throughout surgery, all epidural and muscle motor evoked potentials were stable. Postoperatively the patient’s strength was at his preoperative baseline and has since begun to improve.
Case 2
A 24-year-old female first complained of intermittent cervical pain 12 months prior to presentation. Originally, the pain was not severe and it did not recur frequently. Over the ensuing 12 months, however, the cervical pain worsened and became more frequent. Eventually the pain intensified and she had difficulty getting out of bed. The patient did not experience any other sensory abnormality. The patient did not complain of weakness or bowel or bladder dysfunction. Secondary to the increasing pain, the patient went to her physician who performed a detailed physical examination and ordered radiological examinations.
On examination the patient exhibited full, symmetric strength in her upper and lower extremities. Pinprick sensation and joint position sense were bilaterally intact in her upper and lower extremities. Her reflexes were normal and her toes were downgoing when the Babinski reflex was elicited. She had normal cutaneous perianal sensation and rectal tone.
Plain films revealed mild scoliosis. A cervical spine MRI with and without gadolinium contrast revealed an intramedullary lesion at C4 to C5 (Fig. 11–2). The lesion homogeneously enhanced and was centrally located within the substance of the cord. The spinal cord was slightly enlarged at these levels. A rostral cyst or syrinx was present. No other lesions were noted on brain, thoracic spine, and lumbar spine MRIs.
She was placed on a tapering dose of oral Decadron (Merck and Company, Inc., Whitehouse Station, NJ) and given oral analgesics for pain relief. She was admitted to the hospital for biopsy and resection of her intramedullary lesion. The patient was taken to the operating room for a posterior approach consisting of an osteoplastic laminectomy, dural opening, and resection of the lesion. Intraoperative pathology was consistent with ependymoma. The dura was closed primarily. Throughout surgery, all epidural and muscle motor evoked potentials were stable. Postoperatively the patient’s strength was at her preoperative baseline.
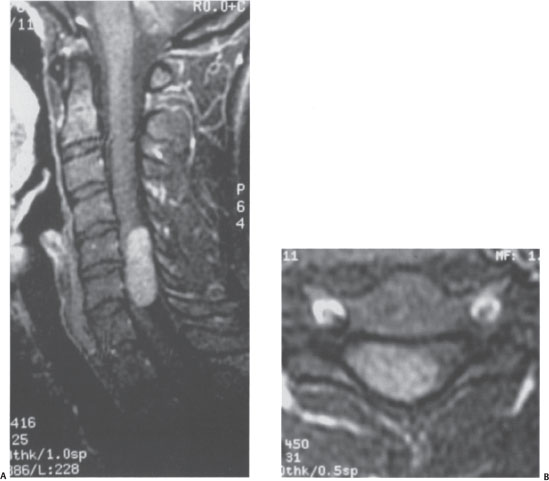
Figure 11–1 Intradural-extramedullary tumor. (A) T1 -weighted sagittal image with gadolinium contrast demonstrates an extramedullary tumor with significant compression of the spinal cord. (B) Axial image confirms the dorsolateral location of this tumor.
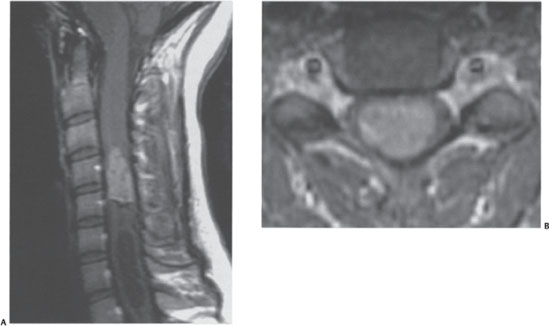
Figure 11–2 Intramedullary tumor. (A) T1 -weighted sagittal image with gadolinium demonstrates an enhancing tumor within the spinal cord associated with a caudal cyst. (B) Axial T1-weighted sequence demonstrates the central intramedullary location within the spinal cord.
 | Background |
Spinal cord tumors represent 4 to 8% of all central nervous system tumors.1–3 The incidence of these neoplasms is approximately one case per 100,000 patient-years.1,3 Spinal cord neoplasms affect both pediatric and adult populations. Overall, spinal cord tumors occur with similar incidences in both males and females.4 Cervical spinal cord tumors occur one third less frequently than thoracic tumors but slightly more often than tumors of the lumbar segment of the spinal cord.3
Intradural cervical spinal cord tumors may originate from either of the two main intradural compartments of the spinal cord, the intradural-extramedullary or intradural-intramedullary compartments. The most frequent intradural-extramedullary lesions arise from nerve roots or the meninges and include nerve sheath tumors (schwannomas and neurofibromas) and meningiomas. Intradural-intramedullary lesions that arise from the substrate of the spinal cord include astrocytomas, ependymomas, oligodendrogliomas, and gangliogliomas.5 Intramedullary cavernous malformations and hemangioblastomas are also observed.5 Less commonly, metastatic disease, germinoma, or lymphoma presents in the cervical spinal cord.3
The most common intradural-extramedullary lesion is the nerve sheath tumor. Nerve sheath tumors account for ~30 to 40% of spinal neoplasms.3,4 Males are affected slightly more often than females.6–8 Patients may present at any age but generally present in their fifth or sixth decade of life.7,8
Spinal nerve sheath tumors can be classified as either schwannomas or neurofibromas.9 Schwannomas are composed of differentiated neoplastic Schwann cells.10 On gross inspection, a schwannoma grows on the periphery of the nerve root. Neurofibromas are composed of Schwann cells, perineural-like cells, and fibroblasts.10 In the neurofibroma, the tumor diffusely expands the substance of the nerve root resulting in the root’s plexiform enlargement. Malignant spinal nerve sheath tumors are rare, representing 1 to 3% of spinal nerve sheath tumors.7,11
Spinal nerve sheath tumors originate from the dorsal spinal nerve root more frequently than the ventral root.8 As a result, these lesions predominantly occur dorsally to the spinal cord. Cervical nerve sheath tumors frequently present with an extradural component and may expand extraforaminally outside the spinal canal.8 This is in contrast to thoracic and lumbar nerve sheath tumors. This characteristic of cervical nerve sheath tumors has been attributed to the relatively short length of the intradural spinal nerve root at the cervical levels compared with more caudal levels.8 Intramedullary extension is rare but may occur.7
Meningiomas may also occur as intradural-extramedullary masses in the cervical spinal cord. Approximately 20% of spinal neoplasms are meningiomas.3,4 In contrast to nerve sheath tumors, meningiomas are three times more prevalent in females than in males.6,12 As with nerve sheath tumors, these lesions generally present in patients aged 40 to 60 years.13,14
Meningiomas are neoplasms thought to derive from arachnoid cap cells and are composed of neoplastic derivatives of these cells.15 These lesions generally present as a firm, encapsulated, and well-demarcated mass. Infrequently the lesions may grow as a flattened, en plaque mass. Meningiomas are generally low-grade lesions and atypical, and malignant meningiomas are infrequently observed.
Cervical and cervicothoracic spinal cord meningiomas occur less frequently than thoracic meningiomas but more often than lumbar tumors. In 50% of cases, meningiomas present laterally to the spinal cord. Meningiomas are located ventral to the spinal cord in ~25% of cases and dorsal to the spinal cord in 25% of cases.13,14 Meningiomas are generally strictly intradural lesions but in 10% of cases extradural extension exists.13,14
Intradural-intramedullary neoplasms that originate from neuroepithelial tissue such as astrocytomas, ependymomas, oligodendrogliomas, and gangliogliomas are the most prevalent intramedullary tumors. Neuroepithelially derived intramedullary spinal cord tumors (IMSCTs) arise from the cellular constituents of the spinal cord and account for 25% of primary spinal cord tumors.3,4 Males and females are similarly affected.16,17
Intramedullary tumors may occur in all age groups but the prevalence of each intramedullary lesion varies significantly with age.5 Overall, the most common intramedullary neoplasms include astrocytomas, ependymomas, and gangliogliomas.5 When patients are segregated by age into pediatric and adult populations, a different prevalence of each tumor is observed. In patients less than 21 years old, astrocytomas predominate and represent 40% of the intramedullary lesions.18 Gangliogliomas are the second most frequent neoplasm (30%) followed by ependymomas (15%). Miscellaneous intramedullary tumors represent the remaining lesions. In adult patients, however, ependymomas are observed most frequently (50%).5 Astrocytomas are encountered 25% of the time and gangliogliomas less than 10%. Fortunately, the majority of IMSCTs are low-grade lesions or have a benign histology. High-grade, malignant lesions such as anaplastic astrocytomas and glioblastomas have a prevalence of 10% in pediatric and adult patients.5
IMSCTs are intrinsic to the cord and often diffusely widen the cord, displacing normal ascending and descending spinal tracts to the periphery. Neither astrocytomas nor ependymomas are encapsulated. Astrocytomas are infiltrative lesions and infrequently possess a well-defined border with normal tissue. Ependymomas are generally more circumscribed in their appearance, however. Both may present with syrinxes or cysts, although ependymomas do so more frequently.
Other lesions may present infrequently as intradural-extramedullary or intradural-intramedullary lesions. These lesions may be neoplastic, vascular, infectious, or inflammatory. Hemangioblastomas and cavernous malformations account for ~5% of intramedullary lesions and infrequently can be found extramedullary in association with nerve roots.5,19–22 Metastatic lesions, germinomas, and lymphomas can also present as intradural mass lesions.5 Spinal cord arteriovenous malformations (AVMs) and fistulas as well as vascular aneurysms may occur.23 Arachnoid cysts may clinically present with symptoms similar to intradural spinal cord tumors.24 Tuberculosis and bacterial subdural empyemas may occur in the cervical spinal cord as well as inflammatory lesions caused by sarcoidosis, transverse myelitis, and multiple sclerosis.
 | Clinical Presentation |
The clinical presentation of patients with both intradural-extramedullary and intradural-intramedullary tumors is highly variable.7,8,14,16 Some patients may be largely asymptomatic at presentation. Other patients present with vague, nonspecific symptoms. Variable degrees of pain, sensory disturbances, motor weakness, myelopathy, or bowel, bladder, and sexual dysfunction may be observed. Intradural spinal cord tumors may also produce scoliosis, especially in children.18
In patients with intradural spinal cord tumors, pain is generally the most common presenting symptom. The pain may be local cervical pain or radicular in nature. Other sensory disturbances, including numbness, gait ataxia, and disturbances of proprioception, are experienced. Weakness, when present, is generally secondary to compression of the cortical-spinal tracts. Weakness usually manifests first with fatigue followed by the upper motor neuron findings of spasticity, clonus, and hyperreflexia.
Lesion location may sometimes be inferred from patients’ signs and symptoms. High cervical lesions may produce symptoms of weakness and myelopathy in both the upper and lower extremities. Lower cervical and cervicothoracic lesions generally only affect the legs. Lesions based lateral to the spinal cord may produce a Brown-Séquard syndrome, an asymmetric pattern of signs and symptoms from the selective compression of the corticospinal tract, spinothalamic tract, and posterior columns of half of the cord. Dorsal lesions may present with disturbances of proprioception secondary to compression of the posterior columns.
The duration of symptoms prior to diagnosis is highly variable. Symptoms may be present for weeks to months or possibly many years. A prolonged duration of symptoms prior to presentation may indicate that the lesion is a slow-growing, benign lesion. Malignant lesions more commonly present with rapid progression of signs and symptoms.18
 | Radiological Findings and Diagnosis |
MRI with and without contrast enhancement is currently the imaging modality of choice for investigating intradural pathology. Intradural pathology is delineated readily by MRI because intradural tumors have characteristic MRI properties. These characteristic properties include the intensity of the tumors on specifically weighted images, the ability of tumors to enhance after contrast administration, the presence of an associated cyst or syrinx, and derangement of normal spinal cord architecture.
Both intradural-extramedullary and intradural-intramedullary tumors are generally iso- to hypointense to normal spinal cord on T1-weighted images. These neoplasms are usually hyperintense to normal spinal cord on T2-weighted imaging. Most importantly, intradural neoplasms normally enhance after contrast administration. The most common extramedullary lesions such as meningiomas and nerve sheath tumors avidly and homogeneously take up contrast. Intramedullary ependymomas homogeneously enhance, whereas astrocytomas enhance heterogeneously. Due to their strong enhancement after contrast administration, even small intradural tumors may be observed with MRI.
Intradural tumors may have additional imaging characteristics that permit their identification. Intradural-extramedullary lesions typically displace the spinal cord away from the lesion. These tumors typically increase the size of the subarachnoid space on the same side as the lesion. Meningiomas may contain nonenhancing regions suspicious for calcifications or cysts. The dura adjacent to meningiomas may be observed to enhance and present as a “dural tail.” Nerve sheath tumors occasionally show heterogeneous enhancement secondary to the presence of cysts, hemorrhage, or necrosis.9 Intradural-intramedullary lesions cause diffuse spinal cord enlargement that eliminates surrounding cerebrospinal fluid (CSF) signal in the subarachnoid space. Ependymomas are often centrally located and associated with rostral or caudal cysts. Astrocytomas are generally eccentrically observed in the spinal cord.
Additional imaging may be indicated in certain instances. Brain MRI and thoracic and lumbar spinal MRI may be required to evaluate for the presence of additional lesions. Anteroposterior and lateral plain x-rays document the sagittal and coronal balance of the vertebral spine. Computed tomography (CT) may be required to provide additional information about the axial skeleton. As with MRI, CT myelography can be used to demarcate intradural pathology, especially in patients unable to undergo MRI secondary to implants or body habitus. Spinal angiography may be required to definitively rule out intradural AVMs or aneurysms.
Associated Neurocutaneous Syndromes
Several neurocutaneous syndromes may be associated with intradural tumors. Both neurofibromatosis types 1 and 2 (NF1, NF2) can be associated with multiple nerve sheath tumors.25–27 NF2 may also be associated with extramedullary tumors such as meningiomas or intramedullary tumors such as ependymomas. Intramedullary hemangioblastomas may occur in patients with von Hippel-Lindau disease.28,29 Patients affected by spinal cord cavernomas have a high prevalence of multiple lesions and familial forms of the disease.30
The association between intradural tumors and neurocutaneous syndromes is important to recognize. When the presence of a neurocutaneous syndrome is suspected, additional radiological evaluation of the entire neuraxis with MRI is required to identify additional pathology. In addition, patients affected by these syndromes require continual follow-up because existing lesions progress and new lesions are likely to occur. Identifying the presence of a neurocutaneous syndrome requires appropriate referrals for genetic counseling and family screening as well as thorough evaluation of other clinically significant systemic manifestations of the diseases.
Patient Evaluation
The clinical management of patients with cervical intradural spinal cord tumors begins with a careful history and physical. It is important to note the patient’s age as well as clarify the patient’s chief complaint. The nature of additional symptoms and length of time they have been present is important. Determining the existence of a family history of disease is necessary. Physical signs of weakness, sensory disturbances, pain, and myelopathy are investigated and their severity precisely documented.
A thorough review of the patient’s radiographic studies is required. The imaging characteristics of the lesion should be analyzed to look for evidence of calcification or hemorrhage. The location (extramedullary or intramedullary; ventral, lateral or dorsal; central or eccentric) and level of the lesion should be noted. The extent of the lesion and the presence of associated lesions such as a cyst or syrinx are determined. The presence of multiple lesions should be established. Degenerative changes as well as sagittal or coronal spinal imbalance should be recognized.
The information gained from the history, physical, and radiology studies is integrated. Based on this information, a differential diagnosis can be established as well as a plan of treatment. Additional radiological studies such as brain MRI, x-ray films, CT, CT myelography, or angiography may be required to determine if additional lesions are present, help narrow the differential diagnosis, or investigate the axial skeleton. Additional consultations with neurology may be required if any uncertainty about the patient’s diagnosis exists because further workup to rule out demyelinating, inflammatory, or infectious etiologies may be indicated, especially for intramedullary processes. Genetic counseling may be appropriate if a family history is established.
In general, patients presenting with an intradural spinal cord mass require a surgical procedure to, at the minimum, establish a tissue diagnosis. Exceptions do exist, however. If an intradural lesion is asymptomatic and incidentally discovered, clinical and radiographic observation on a biannual or annual basis is reasonable. Any evidence of clinical or radiographic progression would then be evidence that surgery was indicated. Observation or palliative care is indicated in patients with known metastatic disease and a very limited life expectancy, regardless of the presentation.
 | Surgical Management |
Preoperative Planning
The preoperative planning for both intradural-extramedullary and intradural-intramedullary lesions is similar. For each lesion, consideration has to be given to individual patient specific factors. These factors influence the choice of surgical approach. In addition, these specifics determine the proposed extent of resection and whether cervical spine stabilization with internal fixation is required. An arterial line must be used for accurate intraoperative blood pressure monitoring. Planning for electrophysiological intraoperative monitoring (IOM) of evoked potentials is essential and must be coordinated with an appropriate anesthetic technique.
Except for specific instances, a posterior approach is advocated for biopsy and resection of intradural tumors of the cervical spine. Patients infrequently present with radiographic signs of instability indicating anterior or posterior internal fixation, and fusion is initially required. The surgical end point of the procedure is usually gross total resection, although preoperative and intraoperative findings may alter this approach.
Intraoperative Monitoring
Intraoperative evoked potential monitoring has become an integral component of safe, effective surgery for intradural tumors, especially intramedullary lesions.31–35 IOM of evoked potentials can involve monitoring sensory conduction pathways [somatosensory evoked potentials (SEPs)] or motor evoked potentials (MEPs) of the spinal cord. SEPs effectively monitor the dorsal columns of the sensory system. They have often been observed to provide inaccurate information regarding the clinically more important motor systems of the patient, however.36–38 In addition, during surgery for intramedullary tumors, SEPs are often lost after the dorsal myelotomy and are unable to provide any further information. As a result of the deficiencies in SEP monitoring, electrophysiology techniques have been designed for direct interrogation of the spinal cord’s motor system during intradural tumor resection via transcranial electrical or magnetic stimulation.32,34,35,39
Transcranial MEPs travel in the descending motor tracts of the spinal cord, in particular the corticospinal tracts (CSTs) and can be monitored from either the epidural space (eMEPs) or at innervated muscle (mMEPs). Real-time alterations of the amplitude, phase, and latency of MEPs during surgery as well as changes in stimulus intensities required to produce MEPs have been correlated with postoperative neurological outcomes.32,34,35,39–41 This is valuable real-time information about the functional integrity of the spinal cord and the motor pathways. Based on this information, surgical plans can be altered intraoperatively before permanent, clinically relevant motor damage is caused.
The anesthetic regimen is an important consideration for appropriate IOM during intradural tumor resection. Halogenated agents depress transmission of MEPs, particularly at the neuromuscular junction, and should generally be avoided.39 Instead, the anesthetic regimen should be based on propofol infusion (100 to 150 μg/kg/min) and fentanyl infusion (1 μg/kg/h) to achieve a steady level of sedation.34 Nitrous oxide can be added to the regimen as well. Boluses of the agents should be limited so as not to perturb MEP recordings artificially. Paralytics should have short half-lives and only be used during induction.
Surgical Approach and Technique
The approach for the majority of lesions is typically a dorsal approach. Fixation of the skull with the Mayfield head holder (Integra, Plainsboro, NJ) is done after patient intubation and just prior to positioning the patient prone. Contraindications to application of a skull clamp include skull fractures or previous craniectomies in the area required for fixation. In these instances a horseshoe head holder may be used.
After application of the skull clamp, the patient is turned prone onto chest rolls or a Wilson frame (Mizuho OSI, Union City, CA). Anesthesia should immediately verify that the patient is ventilated and that the endotracheal tube has not dislodged. The patient’s head is flexed, recognizing that at least two fingerbreadths should separate the chin from the chest so as not to obstruct the endotracheal tube. All contact points between the patient and the bed are padded. All needles required for electrophysiology recording should be placed and preoperative antibiotics should be given.
After skin incision, minimal soft tissue dissection is used to expose the lamina over the intradural lesion. Care must be taken not to expose too laterally and risk destruction of the facets. In addition, intraspinous ligaments should be preserved. Minimal soft tissue dissection will help prevent postoperative delayed instability and kyphosis. After adequate soft tissue dissection is completed, the spinous process is marked with an instrument and a lateral cervical spine x-ray is acquired to confirm location.
An osteoplastic laminotomy is then completed over the solid component of the lesions. A Kerrison punch is first used to create a small laminotomy at the inferior, lateral edge of the most caudal lamina being removed. Ligamentum flavum is dissected off of the inferior lamina with a small, curved curette. The laminotomy will allow the footplate of a craniotome to be inserted. After positioning the craniotome underneath the correct lamina, it is advanced superiorly through each lamina to be removed. This is repeated on the contralateral side. Care has to be taken so as not to veer into the lateral masses. After the bone has been cut, the intraspinous ligaments attaching the spinous process of the most inferior cut lamina to the more caudal spinous processes are cut. The osteoplastic laminotomy is then elevated by disconnecting ligamentous attachments. The elevated osteoplastic laminotomy is hinged off of the most superior intraspinous ligaments that are connected to the rostral cervical spinous process. Contraindications to using the craniotome beneath the lamina include significant mass effect from the lesion or spinal stenosis.
After the osteoplastic laminotomy, epidural venous bleeding is controlled with bipolar cautery, thrombin-soaked Gelfoam (Pfizer, New York, NY), or Floseal (Baxter, Deerfield, IL) and cottonoids. This bleeding is minimized so as not to contaminate the subarachnoid space with blood products and debris later in the case. Before opening the dura, an ultrasound probe is used to insonate the intradural pathology. Most intradural tumors will be hyperechogenic to the spinal cord. Associated cysts will be hypoechogenic. The laminectomy should be large enough to cover the extent of the solid component of the intradural tumor. Additional lamina can be removed if more exposure is required. It should be noted that the full extent of cysts do not have to be exposed unless the cyst walls are enhancing.
After confirmation of tumor localization, the dura is opened in the midline. This can be done under the microscope. Care is taken to avoid inadvertent violation of the arachnoid membrane at this stage. Tenting sutures are then used to apply gentle traction to the dura to facilitate extension of the dural opening over the lesion. At times, the arachnoid membrane may adhere to the dura and require dissection under the microscope to release it. These adhesions are often encountered during additional surgery for tumor recurrence and after radiation therapy. In addition, acute herniation of the cord through the dural opening is sometimes observed. In this case, rapid decompression of the tumor is required to prevent catastrophic ischemic damage to the spinal cord.
By this point, the microscope should be in the field. Extramedullary lesions should be easily visualized. Lateral or anterior lesions may require sectioning of dentate ligaments or upper cervical nerve roots (C1-2). This releases spinal cord tethering so that gentle traction can be applied on the cord to facilitate tumor resection. Biopsy of the extradural lesion is then completed and sent to pathology for diagnosis. The lesion is then resected. Occasionally small lesions may be removed en bloc after dissection of the tumor from surrounding structures. Generally however, the tumor surface is first coagulated with bipolar cautery and then incised with microscissors. Internal decompression is then accomplished using an ultrasound aspirator or bipolar, regulated microsuckers and micro tumor biopsy forceps. Once sufficient decompression is accomplished so that extensive manipulation of the spinal cord is not required, the remaining tumor and capsule are dissected free of surrounding structures and removed.
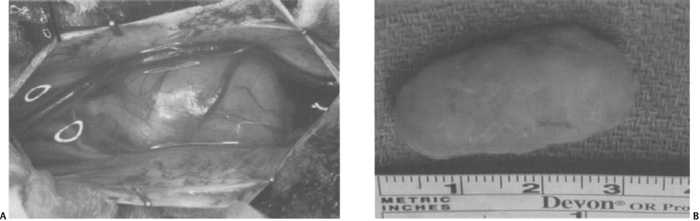
Figure 11–3 Intraoperative photograph for an intradural-extramedullary tumor. (A) Photograph following the opening of the dura demonstrating the dorsolateral position of the tumor. (B) Photograph of the specimen following gross total resection.



