Fig. 15.1
Drawing showing the carotid bifurcation and the removal of plaque at this site by endarterectomy
Carotid endarterectomy is the surgical option for treatment of carotid stenosis. Stenosis that occurs much higher near the intracranial segment of the internal carotid artery cannot be treated with endarterectomy, and carotid artery stenting must be considered [20].
Selection Criteria for CEA
Carotid endarterectomy carries with it the risk of stroke and death along with the risks associated with general anesthesia [21–23]. For this reason the risk to benefit ratio should favor surgical intervention. Recent studies have led to strict selection criteria for patients undergoing CEA. Current selection criteria support CEA for symptomatic patients with severe (>70 %) and moderate (50–69 %) stenosis as well as asymptomatic patients with severe stenosis. Other factors taken into consideration include comorbidities that may increase the perioperative complication rate, history of ipsilateral stroke, and life expectancy [24–26].
Preoperative Testing
EEG and SSEP testing may be performed on a patient prior to the day of surgery. This is not required for accurate intraoperative neurophysiological monitoring of the patient but may be useful in determining whether any abnormalities or asymmetries may be expected in the operating room. The existence of preoperative asymmetries should heighten the awareness of the monitorist of an increased potential for change during cross-clamping especially if there are any residual neurological symptoms following a prior stroke [27]. It is important to utilize the results of preoperative testing for the purposes of planning while remembering that the patient’s intraoperative (post-induction) baselines will be the only data that matter during the monitoring procedure.
Anesthesia for Monitoring of CEA
The anesthetic regimen for intraoperative neurophysiological monitoring of any surgical case is determined based on the modalities to be monitored. For monitoring of most endarterectomies, the anesthetic requirements for SSEP and EEG recordings are to be considered [28]. Anesthesia and intraoperative monitoring is reviewed elsewhere in this volume. When monitoring of the recurrent laryngeal nerve is included in the monitoring protocol, the avoidance of muscle relaxants would also be essential. In the absence of preoperative EEG and SSEP testing, a preinduction baseline can illuminate any asymmetries due to a prior ischemic event. No further importance should be given to preinduction data, as the post-induction baseline will be the data against which changes are judged.
The pattern of EEG will change as the patient proceeds through the various states of anesthesia [28, 29]. Rapid induction, especially with barbiturates, will result in an alpha/beta pattern dominant in the frontal channels. As the stage of anesthesia moves toward the surgical plane, this activity will generalize and then begin to slow. Increases in volatile anesthetics beyond 1 MAC may result in a burst suppression pattern in the EEG, which is not conducive to monitoring EEG. If the EEG is in burst suppression, it is important for the monitoring team to inform the surgeon that EEG monitoring is currently unreliable and then begin to work with the anesthesia team to adjust the regimen to one more permissive of EEG monitoring. Anesthetic protocols may involve the use of minimal inhalants with the addition of a propofol infusion. In many instances it is preferable to have the volatile agent higher as long as it does not exceed 1 MAC and the propofol infusion rate lower. It would be better to avoid a propofol infusion altogether since propofol can lead to a concentration-dependent burst suppression of the EEG. While it is optimal to have data from multiple modalities available when making interpretations, it is worth noting that SSEPs can still be reliably monitored even when the EEG is in burst suppression [30, 31]. Good communication with the anesthesia team prior to the case will help insure that such interruptions in monitoring are kept to a minimum.
Changes in the anesthetic load will also affect the reliability of SSEP data. Symmetric changes in the cortical potential (N20) can be suggestive of anesthetic change, but the possibility of a surgical or peri-surgical cause cannot be ruled out. An asymmetric reduction in the amplitude or latency increase of the N20, however, is suggestive of a clinically significant change over an anesthetic-induced change. It is important that the anesthesia team be aware that changes in anesthetic load (e.g., delivering a bolus) are undesirable, especially near the time of or during an important surgical step.
Monitoring the patient’s physiological status is an important job of the anesthesia team. The neurophysiological monitoring clinician can aid the anesthesia team by correlating change in physiological status with cerebral perfusion. One of the most important functions of the anesthesia team during the procedure is regulation of the mean arterial pressure (MAP). Unlike most spine procedures, the CEA requires that the patients MAP be carefully regulated at different points during the procedure [32]. For example, the MAP is increased during clamp to facilitate collateral circulation but reduced just before unclamping to avoid reperfusion injury. In addition, many patients undergoing CEA have a history of cardiovascular disease and hypertension, which may impede the ability of the arterial system to autoregulate. The consequence of this is that the patient may not tolerate the mean arterial pressure that they are being maintained at by the anesthesia team. Changes in neurophysiological data not correlating with a surgical step may be a result of changes in MAP. This becomes even more critical during both clamping and reperfusion (clamp release) when MAP must be carefully regulated.
Procedure Details and Critical Phases for Monitoring
While continuous neurophysiological monitoring is essential, there are critical phases of the procedure that warrant specific consideration due to the increased risk (Fig. 15.2). Thompson and Talkington [32] provide a good review of the procedural details of carotid endarterectomy. For the purposes of intraoperative monitoring of the procedure, it is important that the monitorist establishes quality baseline data for all modalities monitored after induction but well before cross-clamp. Premedicated baselines should be considered when possible solely for the purposes of revealing any preoperative asymmetries. At least a post-induction 10-min pre-clamp baseline should then be established for the purposes of comparing testing results throughout the procedure [33].
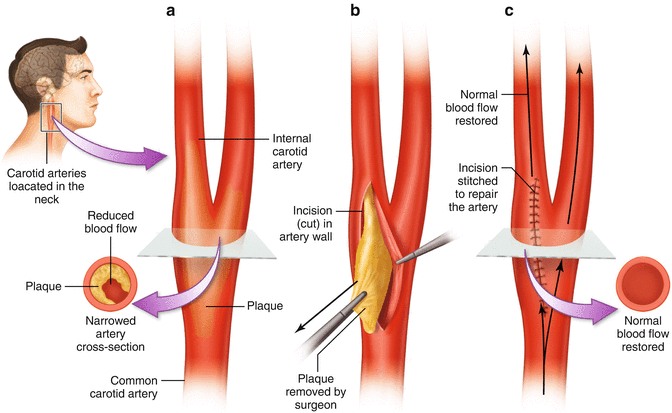

Fig. 15.2
The surgical steps of carotid endarterectomy
The first critical event is administration of heparin. Heparin, an anticoagulant, is given prior to carotid cross-clamp for the purpose of preventing thrombus formation that may lead to embolic stroke on reperfusion. By the same mechanism, heparin may re-aggravate any bleeds that may have occurred from aneurysms or other disorders. It takes 4–5 min on average for heparin to raise the active clotting time sufficiently to proceed with carotid cross-clamping.
The next critical event, carotid artery cross-clamping, is likely the reason the surgeon has ordered monitoring to begin with. As you recall, the carotid arteries feed the ipsilateral anterior circulation of the brain. In most healthy patients, the contralateral circulation compensates for loss of blood flow from one carotid artery. This compensation occurs by virtue of collateral circulation through the circle of Willis. A majority of people have an incomplete circle of Willis, of which there are many variants (Fig. 15.3) [34]. Although incomplete, the circle of Willis is still adequate to provide sufficient collateral circulation in most people. There are, however, certain anatomic variants or pathological conditions (including prior stroke) that result in the inability of the contralateral circulation to compensate for a unilateral carotid occlusion such as occurs during carotid clamping [35]. Changes in electrophysiological data that correlate with carotid cross-clamping should be taken as an alarm that collateral circulation is inadequate to perfuse the brain. A further discussion of alarm criteria will be presented below. In order to facilitate endarterectomy, the common, external, and internal carotid arteries must all be clamped. When collateral circulation is judged inadequate by changes in electrophysiological data, the surgeon will place an intraluminal shunt whose purpose is to reroute blood around the clamp maintaining flow to the brain. Due to the increased risk of embolic stroke with shunt placement, the current standard is to shunt selectively as determined by changes in the monitoring data [4, 6, 7]. The anesthesia team must carefully manage the patient’s blood pressure during cross-clamp. In order to support collateral circulation, the blood pressure is elevated above normal pre-clamp levels. Sufficient blood pressure can be titrated by carefully observing electrophysiological data from SSEPs and the EEG. Insufficient perfusion will result in a loss of amplitude from recorded signals providing a functional assay that can be used to determine the best blood pressure for the patient.
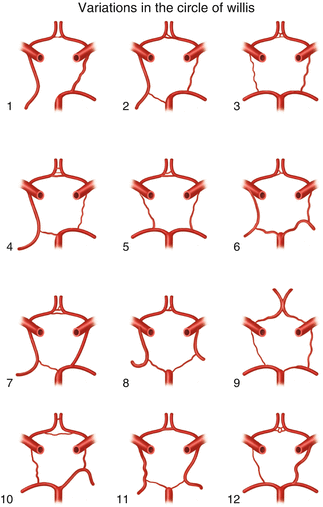

Fig. 15.3
Illustration of 12 variations seen in the circle of Willis
While carotid cross-clamping is largely considered the most critical phase of the endarterectomy procedure by many, reperfusion is the phase during which the patient is most at risk of suffering a stroke. When the carotid cross-clamp is released, particulate emboli are released into the circulation. Most of these emboli are too small to cause a problem, but occasionally larger emboli may become lodged in a smaller vessel creating an obstruction [36]. If the obstruction occurs in a cerebral vessel, the resulting ischemia will likely be detectable as a change in SSEP or EEG data prompting intervention. A subcortical obstruction, however, will likely go undetected by routine monitoring modalities. Figure 15.4 shows an example of a clamp-related change in SSEP and EEG data and recovery of these data following insertion of an intraluminal shunt.
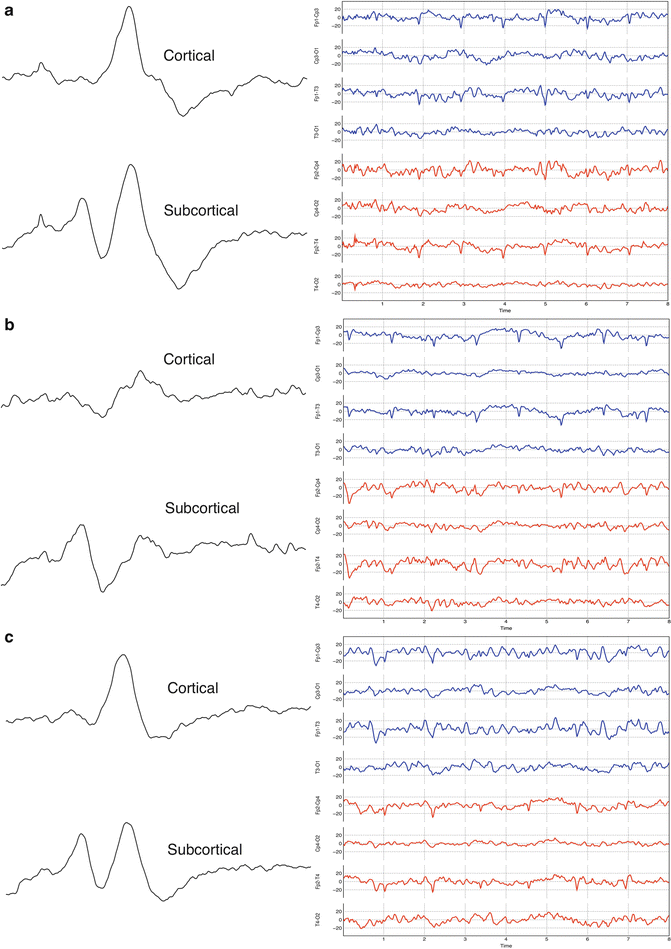

Fig. 15.4
Clamp-related SSEP and EEG change (a) SSEP and EEG baseline data established prior to carotid cross-clamp. (b) Data taken immediately after carotid cross-clamp showing amplitude reductions in the left cortical SSEP and left EEG. Note no change in the subcortical SSEP data. The generator of this potential is supplied by the posterior cerebral circulation. (c) Data taken after shunt placement showing recovery of all amplitudes
Reperfusion injury may occur secondary to a condition known as cerebral hyperemia [37]. Hyperemia can happen in any organ and is the result of too much blood flow. Hyperemia commonly known as reactive hyperemia may occur after a period of ischemia, which, in the case of CEA, may occur during carotid cross-clamp [38]. Hyperemia may develop in the postoperative period and occasionally develops intraoperatively sometime after clamp release. The increase in blood flow seen in hyperemia may cause an increase in intracranial pressure (ICP) that can compress the brain resulting in injury. Transcranial Doppler is the most useful modality in detecting postoperative hyperemia.
EEG Monitoring
Continuous EEG monitoring is used intraoperatively to assess the adequacy of cerebral perfusion and help determine the need for a shunt during carotid endarterectomy [39]. Intraoperative EEG monitoring for carotid endarterectomy does not necessitate recording as many channels as diagnostic EEG. A minimum of eight channels is required for intraoperative monitoring, while the use of more channels is encouraged [40]. The generator of the EEG signal is the cerebral cortex, and as such only cortical perfusion may be monitored with this modality. Subcortical events, such as embolic stroke, are unlikely to be detected with EEG.
EEG monitoring has the advantage of allowing direct monitoring of cerebral function as opposed to modalities such as stump pressure or TCD that only provide an indirect measure of cerebral function. Only SSEPs have demonstrated equal sensitivity to EEG [16]. The addition of median nerve SSEPs, thus, provides a necessary redundancy to EEG monitoring. Hemodynamic changes that do not affect the EEG can usually be assumed to be clinically insignificant, unless an effect is seen in the SSEP recording. EEG monitoring has largely replaced cerebral oximetry for carotid monitoring; however, oximetry may still be used as an adjunct in some centers. Cerebral oximetry measures regional oxygen saturation from the frontal lobes and primarily samples venous blood [9, 11, 12]. The effect of changes in oximetry on cerebral function must be inferred in contrast to the direct information provided by EEG. The following sections provide technical information on setting up and running the intraoperative EEG for monitoring a carotid endarterectomy. The reader is encouraged to become familiar with professional practice guidelines and position statements [41, 42].
Electrode Placement
Stainless steel subdermal needle electrodes are most commonly used for intraoperative EEG with some centers still opting for cup electrodes. The use of needles facilitates a safe and efficient recording setup without the use of adhesives. Electrodes should have an impedance of less than 5 kΩ. A minimum of eight channels of EEG should be recorded for monitoring of carotid endarterectomy. There are several acceptable montages for EEG monitoring of CEA. Table 15.1 shows one of the more commonly used montages often referred to as the modified double banana. A referential montage refers all active leads to a common cephalic reference (usually Cz). In a bipolar montage, active leads are referenced to each other giving the added advantage of increased specificity or ability to more easily locate the area of change. Since efficiency is required in the operative setting, many monitorists make use of their SSEP scalp leads in their EEG montage. The most important considerations are that the choice of recording sites contains areas from frontal to occipital and that leads are placed symmetrically on the left and right side.
Table 15.1
Modified double banana electrode placement
Left | Right |
|---|---|
Fp1-Cp3 | Fp2-Cp4 |
Cp3-O1 | Cp4-O2 |
Fp1-T3
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|




