Fig. 13.1
Transcranial stimulation over the motor cortex using a single square wave pulse results in a recordable D-wave from an epidural electrode. Pulse train stimulation will produce a volley of D-waves sufficient to depolarize lower motor neurons and subsequent recording of compound muscle action potentials from the distal musculature
Sala and colleagues have shown that D-waves are the strongest predictor of motor outcome [19]. Warning criteria for D-wave monitoring are well established with a 50 % amplitude reduction being significant for permanent motor deficit [20]. D-wave amplitude reductions tend to be gradual allowing time for intervention if the neurophysiologist begins to notice a trending amplitude reduction. Sometimes a simple pause in surgery or change in approach will reverse a mild amplitude decrease. Warm irrigation and hypertension are also often successful treatments. Reduction in D-wave amplitude that does not reach 50 % may be a harbinger for temporary motor deficit postoperatively, but this carries an excellent prognosis for recovery [20]. Latency shifts in the D-wave signal have not been found to be clinically significant.
For complete monitoring of motor function, D-waves should be run continuously during resection with occasional pause in surgery to run a muscle MEP [21, 22]. The criteria for interpretation of the muscle MEP remain presence or absence of the CMAP response and should be interpreted in conjunction with D-wave data. Generally, loss of muscle MEP recordings predicts a subsequent loss of D-wave amplitude if no correction is taken. Loss of the muscle MEP with preservation of D-wave amplitudes will not serve as an indication to abandon the surgery; however this situation will result in a temporary postoperative motor deficit with subsequent recovery [19] (Fig. 13.2). Loss of muscle MEPs with preservation of D-waves is thought to occur due to injury to other descending motor pathways with preservation of the corticospinal tract. The D-wave is generated exclusively by the corticospinal tract, while other descending pathways contribute to the muscle MEP [19]. Injury to these additional motor pathways may be compensated for by the intact corticospinal tract accounting for recovery of motor function in the days, weeks, or months following surgery.
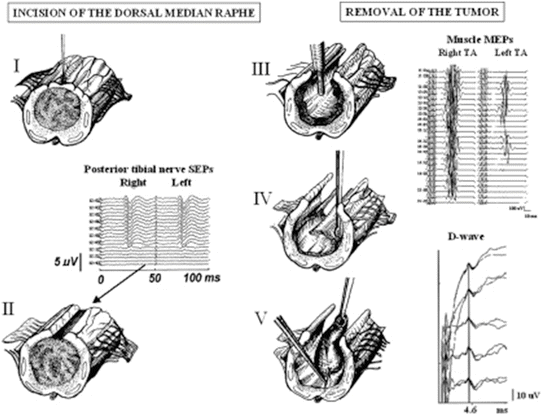

Fig. 13.2
Lower SEP, MEP, and D-wave recordings during removal of an intramedullary tumor. The stages of tumor removal are shown I–V. Lower SEP recordings were lost following midline myelotomy (II) followed by subsequent loss of left lower extremity MEP recordings. D-wave amplitude, however, remained unchanged allowing the surgeon to continue the resection. In a case such as this, a temporary paralysis of surgery can be expected with excellent prognosis for return of motor function
Tethered Spinal Cord
Tethered cord is the name of a constellation of symptoms that occur as a result of abnormal attachment of the spinal cord to other tissues that limits its movement within the thecal sac [23]. There are several causes of tethered cord with the most predominant cause being spinal dysraphism (a type of neural tube defect) [23]. Spinal dysraphisms may be open or closed (occult) (Fig. 13.3). Open dysraphisms include myelomeningocele where the spinal cord and meninges protrude from the child’s back and meningocele where just the meninges are protruding from the back and the spinal cord remains in place. There is occasional association of a benign fatty tumor, a lipoma that attaches to the spinal cord and is covered by skin. This is called lipomyelomeningocele and is a type of occult spinal dysraphism. The various attachments of the spinal cord exhibited in these conditions prevent the spinal cord from ascending to the T12–L1 vertebral level during postnatal growth. This causes metabolic changes in the caudal spinal cord (the conus medullaris) as a result of spinal cord stretch and vascular insufficiency [24]. The cauda equina is also stretched including the sacral nerve roots that mediate bowel and bladder function. See Chap. 2 for anatomical review of the spinal cord and cauda equina.
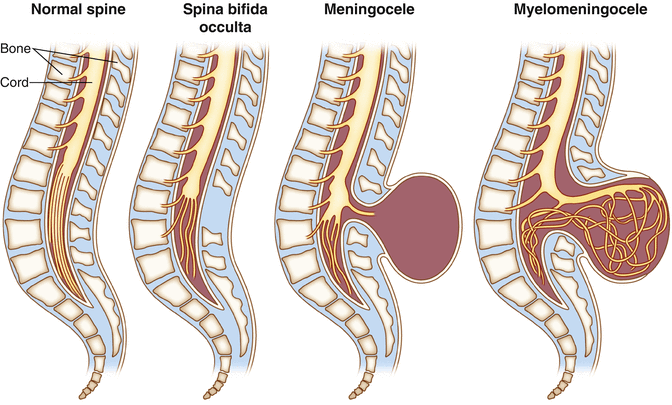

Fig. 13.3
Illustration showing the characteristics of spina bifida occulta, meningocele, and myelomeningocele
Symptoms of tethered spinal cord include back pain, leg pain and weakness, and bowel and bladder incontinence among others [25]. Visual signs in children include a hairy patch over the sacral region, a skin growth, or a depression known as a sacral dimple. If mild, tethered cord may go undiagnosed until adulthood [25]. Diagnosis is made on imaging studies (MRI, CT, myelogram, or ultrasound) [26]. Surgical untethering is the only effective treatment for this condition.
Surgical Procedure
Patient Positioning and Approach
The patient is placed in the prone position, and a skin incision is made over the vertebral segments containing the defect. A laminectomy is performed exposing the dura. The dura is then incised in order to access the lesion involving the conus medullaris and cauda equina.
Anesthesia
The recommended anesthesia regimen is similar to what is recommended above for the removal of intramedullary spinal cord tumors. The avoidance of neuromuscular blockade is of utmost importance for tethered cord surgery due to the importance of both EMG and MEPs for this procedure. 4/4 twitches should be considered essential for effective monitoring.
Untethering
Once the neural elements are reached, the nerve roots are identified and freed from their attachments. The filum terminale is also identified, and if it is contributing to the tethering of the cord is cut. This stage of the procedure poses the most risk to the neural elements. After the cord has been untethered, it is free to ascend in the spinal canal. Injury to the spinal cord can happen at this time if there is any “spring back” of the cord as the filum is cut [26].
Monitoring for Tethered Cord
A multimodality approach is used for monitoring of tethered cord surgery [8, 27, 28]. SEPs, while not generally protective of individual nerve roots, may be used to offer more complete and continuous spinal cord protection than MEPs alone. The proximity of surgery to the conus and the potential for contusion as the cord is released are indictions for SEP monitoring.
The greatest risk during the procedure is to the nerve roots of the cauda equina. Spontaneous EMG is used to monitor the activity of the nerve roots during the procedure [27]. Similar to the use of EMG for other surgeries of the spine, single CMAP responses or small bursts are not clinically significant and may be used by the surgeon to guide the dissection. The use of an audio monitor for EMG is highly suggested for this purpose.
Triggered EMG is used to help the surgeon identify functional nerve roots [28, 29]. Often the surgeon is unable to visually identify nerve roots in the presence of tissue adhesions or a lipoma and will rely on electrical stimulation to discriminate between neural and nonneural tissue [30]. Furthermore, stimulation of the nerve roots can help identify their level of origin and whether or not they are healthy [29]. A healthy nerve root will have a stimulation threshold under 2.0 mA, whereas a pathologic nerve root will have a higher threshold to stimulation. It is important when stimulating that the surgical field be dry and good contact is made between the electrode and the tissue to avoid any current shunting. A bipolar handheld probe is typically used to maximize specificity when stimulating. Electrical stimulation for the purpose of identifying nerve roots or identifying nonneural tissue to excise is often a time of high anxiety for the neurophysiologist as the absence of a response is taken as an indicator that the tissue in question can be cut. This is often the case when the surgeon is seeking to identify and cut the filum terminale to untether the cord [28–30]. For this reason, it is of utmost importance that the surgeon be encouraged to first find and test a functional nerve root so that the adequacy of stimulation and recording can be verified.
Verifying the identity of the nerve roots is only possible if a sufficient number of recording channels are available for the inclusion of multiple muscles [30]. Furthermore, the recording montage must be bipolar (muscles cannot be referenced together) in order to have adequate specificity.
EMG monitoring of the anal sphincter is necessary due to the risk of injury to both the conus and the S4 nerve root [31]. Bilateral recording from the external anal sphincter involves the placement of four needle electrodes in the sphincter muscle. It is important to make sure the electrodes are placed in the muscle itself and not in the skin lateral to the muscle.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







