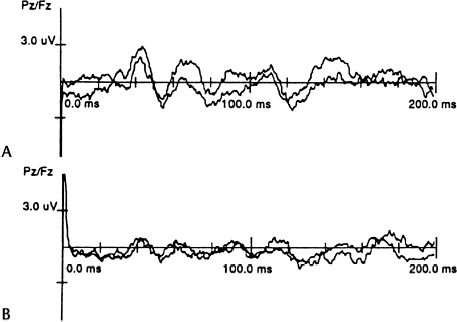
19 Intraoperative Neurophysiological Monitoring of the Lower Sacral Nerve Roots and Spinal Cord Dachling Pang Confusing anatomy is often encountered during operations on complex dysraphic lesions in the lumbosacral canal. It is common to see nerve roots embedded in lipoma or scar tissue, or they may not be easily distinguishable from fibrous adhesion bands. Sometimes nerve roots that are bundled tightly by an abnormally thickened arachnoid can look like a thickened filum terminale. Also, the transition between a functional but structurally deformed conus and an intramedullary lipoma is not always visually apparent. Thus some objective means to identify the sacral nerve roots and the conus is necessary to ensure preservation of these neuronal structures. In addition, in some cases of complex transitional lipomas, the tip of the conus is tautly suspended by low sacral roots that are short, stout, and fibrotic. An assessment of their functional integrity is useful for determining whether dividing them, to complete the untethering process, would lead to unacceptable loss of sphincter function. The first sacral and lower lumbar roots are recognized readily by intraoperative nerve stimulation while palpating for contractions of the respective segmental muscle groups through the surgical drapes. Identification of the lower sacral roots and functional quantification of these roots and their corresponding medullary connections, however, require some objective assessment of perineal sensation and sphincter function. The assessment of evoked responses generated by directly stimulating parts of the sex organs, urethra, and anal canal constitutes the mainstay of sensory monitoring of the lower sacral segments. Monitoring of such responses is most useful when the distal conus or dorsal nerve roots are being rather strenuously handled, as in certain difficult resections of large transitional lipomas or during removal of the median fibrous sleeve of a type I split cord malformation. The latency and amplitudes of the waveforms are exquisitely sensitive to structural deformation and ischemic changes to the central sensory pathways. Sensory evoked response monitoring is less useful in the identification of sacral sensory roots because the responses are generated by end organ stimulation. Cortical responses generated by direct dorsal root stimulation give much less predictable waveforms, which are not stable enough for foolproof identification purposes. The peripheral nerves that supply the bladder, anal canal, and perineal skin, all potentially available for stimulation, are divided into three main groups. Standard recording of the cortical evoked response is made by 5 mm silver or gold-plated cup electrodes or dermal needle electrodes sutured to the scalp. The electrode impedance should be kept below 2000 Ω. The active recording electrode is placed in the midline, ∼2 cm behind the Cz electroencephalographic recording site according to the International 10–20 System of Electrode Placement. This has been demonstrated to give maximum cortical response on stimulation of the penile and clitoral skin. The reference electrode can be placed at several sites, although the forehead (FPz) is convenient and gives a good waveform. Stimuli are delivered at a rate of 3.5 to 5.0 per second, with ∼2.5 to 3.0 times the threshold intensity. The recording console consists of high- and low-frequency filters to keep the band pass at 30 to 1000 Hz. The sensitivity of the signal amplifier is usually set at 2 to 10 µV per division.17 About 250 to 350 responses are averaged to ensure reproducibility of the reading, but weak and unstable signals from severely damaged conuses may require up to 1000 responses to generate an interpretable waveform.18 The most commonly used form of pudendal nerve evoked response utilizes stimuli applied to the sensory domain of the dorsal genital nerve. In the male, the dorsal nerve of the penis can be stimulated either bilaterally or unilaterally using 5 mm cup electrodes placed 2 to 3 cm apart at the base of the penis, with the cathode proximal to the anode. Stimuli up to 3.0 or 3.5 times threshold are well tolerated. In the female, the dorsal nerve of the clitoris is stimulated by 5 mm cup electrodes or fine dermal needle electrodes fixed bilaterally to the cleft between the labia major and labia minor. The anodes are placed adjacent to the clitoris bilaterally and the cathode ∼2 cm posterior to the anode.17 The averaged cortical pudendal evoked response has a similar morphology as the responses obtained from stimulation of the posterior tibial or peroneal nerve. The response has a fairly characteristic “M” pattern, with an initial positive deflection followed by a constant negative, positive, negative, positive waveform.17 Injury to the S2–4 roots or cord segments is manifested by lengthening of the P1 latency and decreased amplitude of the triphasic waves (Fig. 19.1). Cortical evoked responses of very similar morphology and latencies can be obtained using stimulating electrodes embedded in a catheter inserted into the bladder. The catheter has a balloon at its tip, which can be pulled back snugly for anchorage. The location of the urethral electrodes can be kept reasonably constant to eliminate movement artifacts and interference. Electrode-bearing catheters can also be inserted into the anal canal for measurement of anal evoked responses. The catheter is anchored by double balloons, the inner one within the anorectal junction and the outer one wedged at the anal verge. The cortical anal responses do not differ from the urethral responses or the pudendal dermatomal responses.19 Evoked responses can be recorded by electrodes placed on the skin over the spine in humans. They reflect the afferent volley traversing the dorsal columns. The responses progressively increase in latency at more rostral recording locations. Spinal evoked responses are relatively easy to obtain in children, but the amplitudes and waveform definition decrease with age, such that by midteenage years, these responses are more difficult to obtain, as in the case of adults. The response over the mid- to lower lumbar spine consists of an initially positive triphasic potential, representing the volley as it ascends the cauda equina. Over the caudal thoracic spine, the response consists of an initially positive, predominantly negative triphasic wave, the negative component of which has several peaks or inflections.18 The initial portion of this response arises in the intramedullary continuation of the dorsal root fibers, and the subsequent portion reflects synaptic activity concerned with local reflex mechanism rather than the propagation of the response to more rostral cord levels. From the midthoracic to the cervical levels, the response consists of small triphasic potentials that are difficult to follow, presumably arising from multiple ascending pathways including the dorsal and dorsolateral columns.18
Sensory Monitoring of S2-S4 Segments
Anatomy
Cortical Sensory Evoked Response
Pudendal Dermatomal Evoked Response
Urethral Evoked Response
Anal Evoked Response
Spinal Evoked Response
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







