Intraoperative Radionuclide Localization for Osteoid Osteoma and Osteoblastoma of the Spine
Brian J.C. Freeman
Samuel W. Judd
ABSTRACT
Osteoid osteomas and osteoblastomas are relatively rare bone-forming tumors that infrequently affect the spine. Complete surgical excision of the nidus is a prerequisite for curative treatment. However, intraoperative localization of the small nidus is often difficult. Incomplete resection leads to local recurrence with persistent pain, requiring revision surgery. As a result, surgeons often resort to wide local excision to ensure complete excision. This may result in long surgery times, significant blood loss, spinal instability, and subsequent need for posterior instrumentation and fusion. We describe a series of nine cases in which an intraoperative gamma probe was used in conjunction with intraoperative fluoroscopy to assist with localization of the primary tumor and to confirm complete excision of seven osteoid osteomas and two osteoblastomas affecting the spine. The technique allows accurate localization, a less-invasive approach, less bone resection, less resultant instability, less blood loss, and perhaps most important, confirmation of complete excision of the tumor.
Osteoid osteoma is a benign tumor of bone first described by Jaffe in 1935 (1). It consists of a small nidus (up to 15 mm in diameter) of highly vascularized osteoblasts producing osteoid surrounded by dense sclerotic bone (2,3). Osteoid osteoma can occur in cancellous or cortical bone and has been described in all four limbs and the axial skeleton (4,5,6). Patients typically are first seen between the ages of 10 and 25 years (7), complaining of night pain that is relieved by nonsteroidal antiinflammatory drugs (NSAIDs) or salicylates (8). It has been proposed that this typical pain is related to production of prostaglandin by the nidus (9,10). Osteoid osteoma of the spine is less common than that of the long bones, accounting for just 10% of cases (11). It usually affects the posterior elements of the spine, including the lamina, pars interarticularis, pedicles, and transverse and spinous processes (4,8). A handful of cases originating from the vertebral body have been described (12,13). Spinal osteoid osteomas cause axial back or neck pain, depending on location, but may also cause radicular pain, paraspinal muscle spasms, and reactive deformity, notably scoliosis (14,15).
Osteoblastomas are histologically very similar to osteoid osteomas, but are larger, with a nidus bigger than 15 mm in diameter, and behave in a more aggressive way. They may not necessarily have a well-defined sclerotic border and do not always occur with pain that is responsive to salicylates and NSAIDs (16,17,18). Apart from this, they can be regarded in a similar light as osteoid osteoma, and the management principles and difficulties are the same (2,7,18,19).
Curative treatment of osteoid osteoma and osteoblastoma involves the complete surgical excision of the nidus. Incomplete excision results in local recurrence, requiring further surgery (19,20). As a result, to ensure complete excision, many surgeons previously used en bloc resection, potentially leading to spinal instability and the need for posterior instrumentation and fusion (21).
In recent years, various minimally invasive treatments have gained popularity, including computed tomography (CT)-guided percutaneous procedures with intralesional drill excision (22,23), or radiofrequency thermal ablation (24,25,26). However, both techniques have been associated with relatively high rates of recurrence (24,27,28,29).
Both the vascular nature of the nidus and the rapid bone turnover surrounding it mean that osteoid osteomas and osteoblastomas concentrate radionuclide. This in turn makes them readily identifiable with nuclear scintigraphy (21). Rinsky (30) observed this and wrote an influential article on the use of intraoperative radiopharmaceuticals to assist with localization of such tumors.
The use of radionuclides for intraoperative localization of tumors has steadily increased over the past 15 years at our institution (31,32,33). By using the appropriate radiopharmaceutical together with a suitably designed detector system that can be sterilized for use during surgery, it has been possible both to localize the tumour and to confirm complete clearance of the nidus, thereby eliminating the risk of recurrence.
CURRENT TECHNIQUE
All patients have a conventional workup with plain-film radiography, computed tomography, magnetic resonance imaging, and a preoperative 99mtechnitium bone scan to identify and localize the lesions (Figs. 17.1 and 17.2). A prerequisite for use of the intraoperative probe is a positive preoperative isotope bone scan.
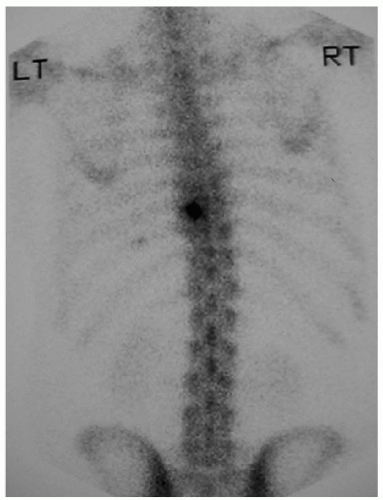 FIGURE 17.1 Radionuclide bone scan showing increased uptake posterior elements on the left in T-9 vertebra (Case 5, Table 17.1). Histology confirmed osteoid osteoma. |
On the day of surgery, 600 MBq of 99mTc HMDP (hydroxy-methylene-diphosphate) is administered intravenously 3 hours before surgery with appropriate reductions in dose for children (34). The surgical team gown with lead aprons. The patient is anesthetized and placed on a radiolucent operating table in the appropriate position. Fluoroscopy is used to confirm the appropriate anatomic level, and a permanent mark is made on the skin. The patient is then prepared and draped. An incision is made, and the posterior elements of the spine are exposed.
We use a 5-mm-diameter cadmium telluride (Cd Te) solid-state detector (Radiation Monitoring Devices, Watertown, MA, U.S.A.) that has been sterilized in ethylene oxide (Fig. 17.3A). The nuclear probe converts gamma radiation directly into an electrical signal, read by a battery-operated digital counter and rate meter (Fig. 17.3B). The sterile probe is passed to the surgeon. The correct operation of the probe system is checked by directing the detector over an area of known high uptake, for example, the urinary bladder. The probe is then used to scan the area containing the lesion, and the counts per
second (cps) are recorded. After this, the posterior elements of the normal bone adjacent to the site of the lesion are scanned and the cps recorded to provide a “background count” for direct comparison. The tumor is then excised (Fig. 17.4



second (cps) are recorded. After this, the posterior elements of the normal bone adjacent to the site of the lesion are scanned and the cps recorded to provide a “background count” for direct comparison. The tumor is then excised (Fig. 17.4
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







