Fig. 15.1
Screenshot of a right temporal high-grade glioma during combined sononavigation. The preoperative contrast enhanced MRI images are seen in the left panel (dual anyplane view). The central panel shows the US images superimposed on the corresponding MR images. The tumor is seen to be hyperechoic. The brainstem and ipsilateral temporal horn can be appreciated on both US and MR images to facilitate orientation. Also note the brainshift depicted on the US images (white arrow) as evidenced by the change in position of the tentorial edge between the US and the MR images
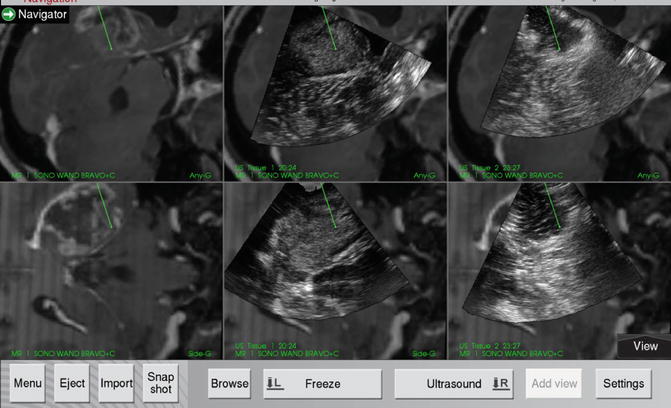
Fig. 15.2
Screenshot of the same patient as in Fig. 15.1 depicting various stages of the resection. Left panel shows the preoperative MR. Central panel shows the pre-resection US overlaid on the corresponding MR. The right panel shows the post-resection US overlaid on the MR. Note the resection cavity in the last scan with a hyperechoic rim (artefacts due to blood in the wall of the cavity). Any suspicious residue can be identified and reached using the navigator
It must be reiterated that this 3D US is not truly real-time. This means that the 3D US is first acquired and the images are reformatted and then subsequently used to navigate. Operating while a 3D scan is in progress is not possible. Solutions for real-time 3D US are available and used in gynecology; however its role in neurosurgical guidance remains to be established (Bozinov et al. 2011).
Accuracy and Impact of 3D US
The Trondheim group showed (using meticulous histological correlation of biopsies with the US as well as MR images) that this system using navigable 3D US was as good and reliable as navigated MR for delineating high- and low-grade gliomas as well as metastases (Unsgaard et al. 2005). They reported high specificity and positive predictive values (PPV) indicating the safety of using this system for guiding resections, but they also found a low negative predictive value implying that when the IOUS was negative there was a possibility of tumor left behind. Improvements in image resolution capabilities in the future are expected to resolve these issues. Interestingly, the same study also found a higher PPV for low-grade gliomas. In a follow-up study the same group from Trondheim evaluated the accuracy of the system during the resection (in the subsequent phase of the surgery) (Rygh et al. 2008). They found that due to imaging artifacts imparted by blood and other changes in the adjacent tissue due to handling, the specificity and PPV dropped. Careful attention during hemostasis and tissue handling are essential to ensure optimal image quality.
With respect to the clinical impact of the 3D US system, it was effectively used in an unselected consecutive cohort of high-grade gliomas (Solheim et al. 2010). In this cohort the authors were able to achieve acceptable results (37% gross total resections [GTR] with 13% morbidity). They also noted that the system was routinely used in a majority of their surgeries and by a wide range of surgeons (including residents) attesting to the ease-of-use and wide applicability of the technology. In a subsequent study the same group showed that survival in GBMs improved in the years after the routine introduction of the SonoWand system (Saether et al. 2012).
Assessment of Utility of Intraoperative Ultrasound
The goal of using IOUS or any other intraoperative imaging tool, is to obtain accurate, comprehensive, and easily interpretable, and usable images, as frequently as needed. This is the endpoint or “gold standard” with which to measure the utility of the tool. There are many aspects that need to be assessed when determining the overall utility.
Efficacy: Efficacy implies the performance of the technology under ideal circumstances. For the ultrasound related imaging tools, this heavily depends on the resolution of the probe being used, and is more or less not modifiable beyond a certain limit. Given ideal conditions the image resolution of the probe reflects its efficacy. It must be borne in mind though, that “ideal” conditions, may not always be present especially in real-life situations where more often than not this is the case. Conditions may be suitably modified to improve the efficacy and this is an important learning step when initiating oneself to the technology. For example, ultrasound images may be compromised by suboptimal acoustic coupling and artifacts. Ensuring a vertical cavity (by intelligent patient positioning), using acoustic coupling (saline or gel) and eliminating air bubbles, and paying heed to hemostasis (avoiding extraneous hemostatic agents) can improve the image quality and thereby the efficacy of the tool. The efficacy of the probe will also determine the accuracy of the images. The measure of accuracy is reflected in the sensitivity, specificity, and predictive values of the imaging tool. This requires histological correlation. Fortunately, dedicated studies have addressed this issue with respect to the navigable 3D US system (Unsgaard et al. 2005) Modes/Functions: Different imaging modes can be used either with the same probe or using a combination of probes. 2D-scans are the commonest mode of use and provide real-time streaming images. These are useful for initial scanning and getting an overview (so called “bird’s eye-view”) of the field of interest. It helps orient oneself to the field. In the navigable US system this can be used superimposed on the corresponding MR images and provide increased confidence and comfort in orienting the surgeon (especially in the initial phase of the learning curve). As described above, there are certain significant limitations of 2D scans in the context of intraoperative image-guided surgery. The 3D US mode seems to overcome some (though not all) of these limitations. For one, it permits a volume imaging rather than just a planar imaging. Combination with navigation technology (navigable ultrasound) then allows representation of the 3D volume in various planes as desired (multiplanar imaging), a very important and crucial step in planning and guiding surgical procedures. Besides the ultrasound imaging special functions which are particularly useful are the Power Doppler (PD), colour flow imaging. These allow visualization of important vascular structures, knowledge of which can be crucial during surgery. The PD function in particular permits high resolution angiography intraoperatively and coupled with navigation is a very handy tool (Fig. 15.3). Scope of use: The scope of application of the US reflects the range of procedures it could be used for. This, in turn, is a function of the ability to insonate the pathology/region of interest. It could range from (though not limited to) tumors, inflammatory processes, and cysts to vascular lesions (hematomas and malformations), both of the brain and spinal cord. It can be used for image-directed procedures such as biopsies (frameless biopsy), catheter, shunt placements, and delivery of local therapies. It can also be used for identifying deep-seated lesions (localization) and controlling the resection of many intra-axial tumors (resection control). Effectiveness: This measures the usefulness of the technique in daily real-life situations and in that sense truly reflects the “practical utility” of the tool. Though difficult to establish objectively, it is a combination of the efficacy, modes of function, and scope of application which governs the overall utility. It may so be that a technique or tool has excellent efficacy (in ideal situations); but if the ideal conditions are very unlikely most of the times, the tool would have limited utility and hence is not very effective. Even if it may be possible to have ideal circumstances, the modes of function may be limited and hence scope of use restricted to a small percentage of routine procedures. Again, though efficacy would be high, applicability would be low and hence the overall utility would be limited. For example, if there is very high-resolution probe but the footprint is too large, it may not be possible to use it in smaller craniotomies. This could be circumvented by having a range of probes with different footprints. Again, the resolution of a 2D scan could be excellent but it may be limited in facilitating real-time surgery by physically interfering with the surgical instruments in the field. In this case it may be preferable (and therefore more useful) to acquire a 3D volume which can be registered and then navigated, permitting removal of the probe and introduction of the surgical instruments with freedom to manoeuvre them as desired. So, a combination of accessories and functionalities, each individually efficacious in certain situations may improve the overall effectiveness of the tool. For a technology to be acceptable, it should have easy accessibility, widespread applicability, and reasonable efficacy. This would ensure a better overall efficiency (effectiveness or output in relation to costs or input). It is always preferable to have an efficient tool, rather than a very efficacious one with limited efficiency. Surgeon Comfort: This is an very subjective parameter. The surgeon’s comfort with the tool is related to his/her experience with the particular tool. It is especially so with IOUS where there is a steep learning curve. This is primarily because neurosurgeons in general are more familiar with MR images than US images of the brain. As mentioned earlier, navigable 3D US overcomes most of the perceived drawbacks of 2D US. Repeated use and experience with the application (as with any surgical tool or technique) is imperative to reach a “comfort-zone”.
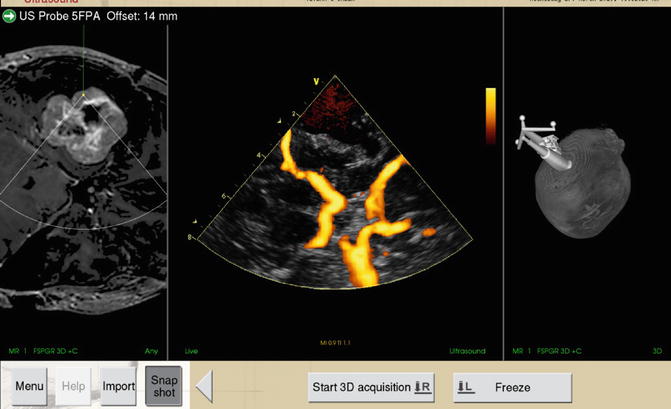

Fig. 15.3
Intraoperative Power Doppler angiography. Screenshot of a case of right temporal chondroscarcoma (preoperative MR images seen in left panel). The centre panel depicts the intraoperative angiogram obtained using the PD function. Right panel shows the 3D surface view. Note the excellent delineation of the circle of Willis. With kind permission from Springer Science+Business Media: Acta Neurochirurgica:Usefulness of three-dimensional navigable intraoperative ultrasound in resection of brain tumors with a special emphasis on malignant gliomas. Ahead of print DOI:10.1007/s00701-013-1881-z. Moiyadi AV et al, Figure 2
Objective assessment of utility of surgical adjuncts is very difficult and scarcely reported. Unlike regulations for new medicinal drug which mandate phase 1 and 2 studies to establish efficacy and safety of the drug prior to phase 3 clinical studies, no such stipulations dictate the introduction of new surgical techniques. Most clinical trials and studies dealing with surgical adjuncts concentrate on other outcome measures (extent of resection, immediate perioperative outcomes, or survivals). Few studies have been reported dealing specifically with efficacy and utility.
Machi et al. (1984) had objectively assessed the role of IOUS in brain and spinal surgery and reported that it was useful for localization of the lesion, for delineation of tissue features as well as assessment of spatial relationships. This was a valuable though concise and incomplete assessment. Kumar et al. (1993) have reported a three point scoring system to assess the utility of IOUS. For cranial cases this score assessed the concurrence of the surgical plan with or without the IOUS by evaluating three parameters viz. location, depth, and planned trajectory to the lesion. The more the discordance in the plan, the higher the score, and better the utility of the IOUS. This was primarily an assessment of the IOUS for the purpose of biopsy of deep seated lesions. The spinal scoring system (also a 3-point score) assessed different parameters (adequacy of laminectomy, adjacent neural elements, and characteristics of the tumor). The authors concluded that IOUS was useful in the cases they studied, although no validation of this score has been reported.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






