Fig. 1
Relationships between tenascin-C levels in the cerebrospinal fluid and World Federation of Neurosurgical Societies (WFNS) grade on admission. Bars, means ± standard errors of the means, r Spearman’s rank correlation coefficient, ns not significant
DCI or Vasospasm Versus TNC Levels in the CSF
DCI occurred in 14 patients: Day 5, n = 1; Day 6, n = 5; Day 7, n = 3; Day 8, n = 1; Day 9, n = 3; and Day 10, n = 1. Patients with DCI had significantly higher TNC levels compared with those without DCI at Days 1–6 (Fig. 2a). DSA was performed in 22 patients, and severe vasospasm was associated with significantly higher TNC levels compared with mild or no vasospasm at Days 1–6 (Fig. 2b). Patients with poor outcomes (severe disability, persistent vegetative state and death; n = 10) also had significantly higher TNC levels than patients with good outcomes (good recovery and moderate disability; n = 20) at Days 1–3 (P = 0.002), 4–6 (P < 0.0001), and 7–9 (P = 0.023; unpaired t test).
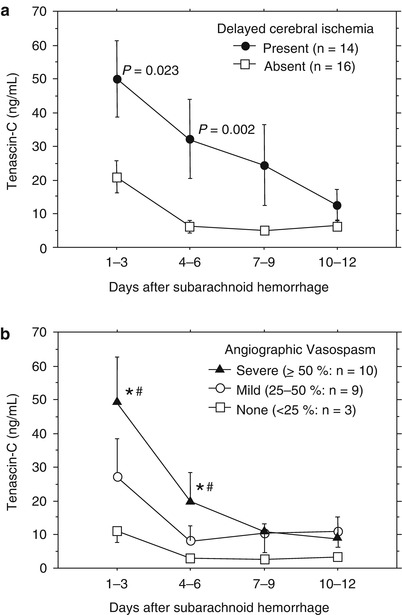

Fig. 2
Relationships between tenascin-C levels in the cerebrospinal fluid and delayed cerebral ischemia (a) or cerebral vasospasm (b). Bars, means ± standard errors of the means, P values in (a), unpaired t test; significantly different from the values in patients with no vasospasm (*P = 0.031, ANOVA); significantly different from the values in patients with mild vasospasm (# P = 0.042, ANOVA)
CSF TNC Levels Stratified by DCI and Vasospasm
When CSF TNC levels at Days 1–6 were evaluated for each DCI–vasospasm bracket, patients with DCI had significantly higher TNC levels than those with no DCI in both mild and severe vasospasm (Fig. 3).
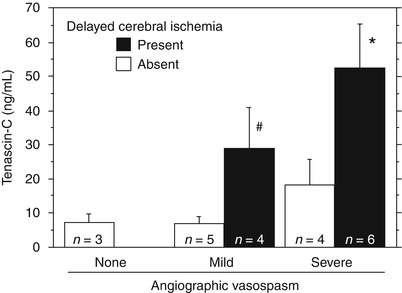

Fig. 3
Tenascin-C levels in the cerebrospinal fluid at Days 1–6 after subarachnoid hemorrhage stratified by delayed cerebral ischemia (DCI) and cerebral vasospasm (severe, ≥50 %; mild, 25–50 %; none, <25 %). Bars, means ± standard errors of the means; significantly different from the values in patients with severe vasospasm but no DCI (*P = 0.011, ANOVA); significantly different from the values in patients with mild vasospasm but no DCI (# P = 0.028, ANOVA)
On multivariate analyses, higher TNC levels in the CSF at Days 1–6 (continuous variable; odds ratio, 1.070; 95 % confidence interval, 1.032–1.109; P < 0.001) and cerebral (angiographic) vasospasm (odds ratio, 4.352; 95 % confidence interval, 1.095–17.301; P = 0.037) significantly predicted DCI occurrence, when age, sex, WFNS grade on admission, aneurysm location, treatment modality, angiographic vasospasm, and TNC levels in CSF at Days 1–6 were used in the analyses.
Discussion
The novel findings in this study are as follows: (1) SAH or initial brain injury that is more severe induces more TNC, which may cause vasospasm and DCI separately or simultaneously; (2) severe vasospasm may cause DCI with more TNC induction; and (3) DCI may occur without severe vasospasm, but by vasospasm-unrelated causes with TNC induction. These findings support recent research efforts that focus on clarifying the pathophysiology of post-SAH early brain injury as well as vasospasm, and on developing protective strategies against them to improve outcome after SAH.
The expression of TNC, a matricellular protein, is extremely limited in healthy adult tissues, but is induced rapidly (within several hours), ectopically, and profusely by inflammatory or noxious stimuli and disappears with their removal [2]. A previous clinical study reported that higher CSF TNC levels were associated with the development of vasospasm [15], and this study suggested that TNC induction also caused vasospasm-unrelated secondary brain injury, which is supposed to be early brain injury. Although these studies suggest that CSF TNC may be a useful biomarker to quickly diagnose or predict the development of vasospasm and DCI, whether TNC can be a therapeutic target to prevent or treat vasospasm and/or DCI is undetermined.
TNC activates mitogen-activated protein kinase (MAPK) [5, 12], which may cause both vasospasm and early brain injury after SAH [13, 14]. TNC may also stimulate synthesis of proinflammatory cytokines and growth factors, and promote their signaling pathways to MAPK activation via enhancing crosstalk signaling between receptors [6, 10]. Our recent experimental study demonstrated that imatinib mesylate, an inhibitor of the tyrosine kinases of platelet-derived growth factor receptors, suppressed TNC induction and MAPK activation, and prevented both cerebral vasospasm and neurological impairments after SAH in rats [12]. However, we cannot exclude the possibility that imatinib mesylate exerted protective effects via mechanisms other than TNC suppression. Because there are neither inhibitors nor neutralizing antibodies specific for TNC, TNC knockout mice would be useful to demonstrate the exact functional role of TNC in vasospasm or early brain injury after SAH [11].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





