 25
25 
Is There a “Best” Way to Give Mannitol?
BRIEF ANSWER
The only level I recommendation supported by the evidence is the use of high-dose mannitol (at least 1.2–1.4 g/kg) in comatose trauma patients with operative acute subdural hematoma or operative intraparenchymal temporal lobe hemorrhage. Use of mannitol (0.25–1.0 g/kg) to control elevated intracranial pressure (ICP) in severely head-injured patients is a level II recommendation. In this setting, mannitol may be especially effective when cerebral perfusion pressure (CPP) is below 70 mmHg (level III recommendation). Additional level III recommendations include administering bolus doses of mannitol at a rate not exceeding 0.1 g/kg/min, replacing urinary losses of fluid, and maintaining serum osmolarity below 320 mOsm/L and serum osmolar gap below 55 mOsm/L.
Background
Mannitol, an inert six-carbon alcohol of the corresponding sugar mannose, causes cellular dehydration by increasing serum osmolarity. Wise and Chater1 pioneered the use of hypertonic mannitol to reduce cerebral swelling (class III data). Although the use of mannitol in head injury has been recommended as a guideline,2 the optimal use of mannitol remains a complicated issue about which the data are incomplete. Questions persist about such issues as bolus versus continuous administration, ICP-directed dosing versus regularly scheduled boluses, optimal rate of infusion, optimal dosage, fluid replacement of urinary losses, clinical thresholds for treatment, role of serum osmolarity monitoring, and timing of treatment after entrance into the trauma care system. Because of this paucity of data, nearly all recommendations about the best method of mannitol administration in head injury (except for treatment of operative acute subdural hematoma or intraparenchymal temporal lobe hemorrhage in comatose head-injured patients) must be considered level III with respect to both ICP control and clinical outcome.
Literature Review
Mechanism of Action
The original model to explain mannitol’s effects proposed that ICP reduction occurred through an osmotic gradient created between the blood and the brain. This gradient reduced brain water content. Therefore, mannitol therapy has classically been directed toward establishing and maintaining this gradient.3 More recently, however, this paradigm has shifted. The time course of reduction in ICP after bolus mannitol infusion is not explained by its osmotic effects. Experiments in cats showed that the ICP decline from mannitol was greatest 30 minutes after a bolus, even though brain water content remained stable.4 Subsequent laboratory studies showed that mannitol decreased blood viscosity and caused a decrease in the diameter of pial arterioles in a manner similar to te vasoconstricting effect of hyperventilation.5
Although mannitol-induced osmotic tissue dehydration may still play an important role in reducing ICP (class III data),6 mannitol’s primary mechanism of action is its rheologic effect. Mannitol dilutes the blood and increases the deformability of erythrocytes (class II data),7 thereby decreasing blood viscosity. CBF is augmented both by the decreased viscosity and by the favorable effect of the mannitol bolus on mean arterial pressure (MAP). The sudden increase in CBF causes autoregulatory vasoconstriction of cerebral arterioles, decreasing the intracerebral blood volume and lowering the ICP (Fig. 25-1). This vasoconstriction in the presence of maintained CBF may explain the lasting effects of mannitol on ICP (class II data),8 even after mannitol has been excreted.
Pearl
Mannitol’s primary mechanism of action is its rheologic effect. For this reason, mannitol can have dramatic effects on cerebral blood flow (CBF) even in cases in which it has only minimal effects on ICP.
It is important to realize that even in cases in which mannitol does not reduce ICP (much), it can markedly increase CBF through its rheologic effect (class II data).9,10 This augmentation of CBF may have important clinical consequences (class III data).11
Bolus versus Continuous Infusion
No clinical studies have compared bolus to continuous mannitol infusion. Becker and Vries12 presented a retrospective case series of 18 patients treated with either bolus or continuous infusion of mannitol, but no information was given as to which method was used in each case (class III data). James13 reported that, in a series of 60 patients, mannitol boluses of at least 1.0 g/kg always reduced ICP by at least 10%, but smaller doses did not always reduce ICP. James also reported that continuous infusion was successful in keeping ICP below 25 mmHg in 16 of 18 patients (class III data). Marshall et al3 reported that mannitol boluses alone were effective in reducing ICP (class II data).
Arguments in support of bolus dosing are based on the postulated mechanism of action of mannitol; that is, on a rheologically mediated autoregulatory response. Muizelaar et al10 demonstrated that mannitol boluses (0.66 g/kg over 3 minutes) were effective in reducing ICP (class II data). Furthermore, in patients with intact autoregulation, ICP was reduced by 27%, but CBF remained unchanged. In patients with defective autoregulation, ICP dropped by only 4.7%, and CBF increased by 18%. This differential response is consistent with a rheologic mechanism of action for mannitol. The effect on clinical outcome of mannitol bolus treatment for elevated ICP is less clear. In patients in whom ICP was above 20 mmHg, Unterberg et al14 reported significant improvement in ICP and CPP after infusion of mannitol (125 cc of 20% mannitol over 30 minutes), but not in brain tissue oxygenation (PbtO2) or jugular venous oxygen saturation (class II data). No changes were seen in these parameters when ICP was below 20 mmHg. In a separate communication from this same group, Hartl et al15 (class II data) found that mannitolhad little effect on PbtO2.
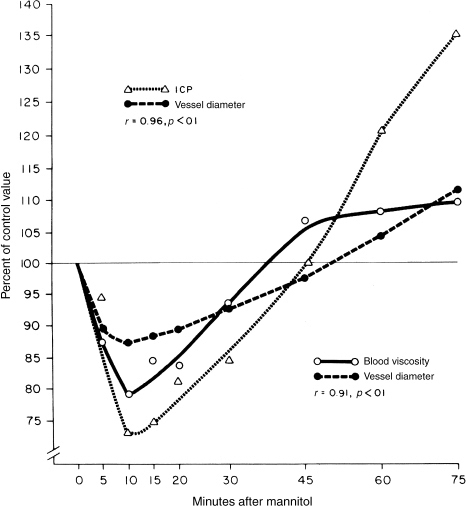
Figure 25-1 Graph showing the effects of mannitol in an experimental model. Changes in blood viscosity, pial vessel diameter, and intracranial pressure are plotted versus time.
Arguments against continuous infusion are based on expert opinion16 (class III data) that prolonged high levels of serum mannitol result in rebound brain edema as mannitol leaks into the interstitium. Experimental evidence in cats has shown that repeated dosing of mannitol leads to an increased accumulation of mannitol in the brain, especially in areas with injury-induced vasogenic edema.17 Becker and Vries12 reported a very high mortality rate in patients in whom mannitol therapy was associated with high serum osmolarity (class III data). This high mortality was probably attributable to high serum mannitol levels. Mannitol can produce acute renal failure (class III data),18 and bolus dosing would presumably prevent chronic exposure of the renal tubule to mannitol.
Rate of Infusion
Very rapid infusion of mannitol has been reported to cause hypotension via decrease in peripheral vascular resistance from vasodilation in skeletal muscle (class III data).19 In a study by Ravussin et al,20 1 g/kg of mannitol infused over 4 minutes (0.25 g/kg/min) to three human subjects caused a drop in MAP of 12 mmHg and a rise in heart rate of 30 beats per minute (both statistically significant) (class III data). The blood pressure returned to baseline within 2 minutes, whereas the heart rate required 15 to 30 minutes to normalize. In a series of six craniotomy patients, mannitol delivered at a very fast rate of infusion (20% mannitol at 60–80 mL over 15–20 seconds, or roughly 0.8 g/kg/min for a 70-kg adult) caused a mean drop in MAP of 30 mmHg. Blood pressure generally returned to normal within 2 minutes when the infusion rate was slowed (class III data).21 The authors suggested that hypotension occurs when the infusion rate of mannitol is ” much more rapid“ than 1.0 g/kg administered over 10 minutes (0.1 g/kg/min). Because sudden hypotension associated with mannitol infusion times of less than 5 minutes (0.2 g/kg/min) has been observed in head-injured patients as well (class III data), Rosner and Coley8 ” prefer 15- to 30-minute rapid drips“; that is, infusion rates of 0.033 to 0.067 g/kg/min. To avoid the risk of sudden hypotension from rapid infusion of mannitol while still preserving its plasma-expanding effects, mannitol should probably be bolused at a rate not exceeding 0.1 g/kg/min, or 10 minutes for a dose of 1 g/kg.
Pearl
To avoid the risk of sudden hypotension from rapid infusion of mannitol, it should be bolused at a rate not exceeding 0.1 g/kg/min, and urinary losses of fluid should be replaced.
Dose
The optimal dose of mannitol has been addressed both with regard to emergency resuscitation and with regard to ICP control in the neuromonitoring unit. Class I evidence supports use of high-dose mannitol (1.2–1.4 g/kg) in the preoperative treatment of acute subdural hematoma. Thus, such treatment should be considered a level I recommendation in this circumstance. Cruz et al22 (class I data) prospectively randomized patients with operative acute subdural hematoma into conventional-dose and high-dose mannitol treatment groups. All patients received an initial mannitol bolus of 0.6 to 0.7 g/kg in the emergency room. After computed tomography (CT) scanning revealed an acute subdural hematoma, patients were randomized to one of two groups. In the conventional-dose group, no additional mannitol was given, regardless of pupillary changes. In the high-dose group, treatment was based on the pupillary exam; patients in the high-dose group without anisocoria received an additional 0.6 to 0.7 g/kg of mannitol prior to operation, whereas patients in the high-dose group with anisocoria received an additional 1.2 to 1.4 g/kg of mannitol. To prevent hypotension from mannitol-induced osmotic diuresis, patients in all groups received normal saline boluses after each administration of mannitol. Preoperative improvement in pupillary widening was significantly better in the high-dose treatment group. The two groups did not differ significantly in the incidence of refractory intracranial hypertension requiring barbiturates or decompressive surgery. A significantly increased likelihood of low cerebral oxygen extraction and brain swelling was seen in the control group. At 6 months, Glasgow Outcome Scale scores were significantly better in the high-dose group.
These same authors have also reported a study of the effects of high-dose versus conventional-dose mannitol in patients with traumatic intraparenchymal temporal lobe hemorrhages.23 In that study, comatose head-injured patients were eligible if they presented with abnormal pupillary widening and normal or elevated blood pressure. Half the patients were randomized to receive a conventional dose of mannitol, or ~0.7 g/kg. The other half were randomized to receive the high dose; that is, 1.4 g/kg. Mannitol infusion was followed by normal saline boluses. As in the above study, the high-dose mannitol patients demonstrated a significantly greater degree of early improvement in pupillary widening and also had higher cerebral oxygen extraction, less frequent brain swelling, and better 6-month outcomes than the conventional-dose patients.
Pearl




