20 Stroke is a major public health illness that is debilitating and expensive.1,2 It requires a team approach to treatment. This team includes a neurologist or stroke specialist, a neurosurgeon, a neuro-interventionalist, a stroke physiatrist, a stroke nurse specialist, a physiotherapist, an occupational therapist, a speech-language therapists, a social worker, and many others. In-hospital stroke care takes place in the stroke unit. Although in most hospitals the stroke units are organized and run by medical specialties (neurologists, geriatricians, internists), the neurosurgeon has a critical role to play in the stroke team. This chapter discusses introductory information on ischemic stroke, and provides basic information on the medical care of the stroke patient in-hospital and through rehabilitation and outpatient management of secondary prevention. It focuses on the neurosurgical aspects of stroke care. Stroke is a sudden vascular syndrome of the brain and can be of four types: ischemic stroke (includes transient ischemic attack [TIA]), intracerebral hemorrhage, spontaneous subarachnoid hemorrhage (SAH), and venous sinus thrombosis. Ischemic forms are the most common; acute ischemic stroke and TIA make up 85% of all strokes in the Western World and 10 to 15% fewer in Asia due to a greater predominance of intracerebral hemorrhage. Intracerebral hemorrhage and atraumatic/aneurysmal SAH make up 15% of all strokes, and venous sinus thrombosis make up less than 1% of all strokes. Ischemic stroke should be thought of as a continuum from the mildest, transient forms—TIA—to the most severe, malignant middle cerebral artery (MCA) syndromes. Ischemic stroke is the end result of a multiplicity of possible underlying causes and shows an exponential incidence with increasing age. By the age of 80 years, one in four persons will have suffered a stroke. Epidemiologically, stroke is not well characterized by its incidence or prevalence because it is an episodic condition that has multiple underlying causes. Rates are best described as events or occurrences. First-ever stroke is the best surrogate term for incident stroke. Prevalent stroke is not a meaningful term and should be avoided. Over the last half century, the age-adjusted occurrence of stroke has been slowly falling. Stroke now occurs at 150 per 100,000 population in Canada,3 which means that within a given population of 1 million persons, it is expected that there will be 1,500 strokes per year. Rates are similar in other Western countries. Stroke mortality has been similarly falling.4 Although stroke is the second leading cause of death internationally, it has dropped to fourth leading cause of death in North America.5 Nevertheless, with a progressively aging population in the developed world, the total number of strokes and hence the burden on health systems is going to rise substantially over the next 25 years. The most important risk factor, quantitatively, for stroke of all types is hypertension.6 Ischemic stroke risk factors vary by stroke mechanism. We can think of risk factors, such as hypertension and risk states, such as carotid artery stenosis. Ischemic stroke can be broadly defined by the mechanism based on the presumed immediate cause of the stroke. There are five groups of causes: (1) cardioembolic, (2) large artery atherosclerotic disease, (3) lacunar or small vessel disease, (4) other uncommon causes, and (5) cryptogenic or unknown cause. Atherosclerotic risk factors include hypertension, diabetes mellitus, smoking, excessive alcohol consumption, elevated cholesterol or other hyperlipidemia, obesity, physical inactivity, hyperhomocysteinemia. Diet and sedentary lifestyle are important contributing factors. Hypertension is the most common risk factor for stroke with an attributable risk approaching 40%, meaning that if hypertension was eliminated, stroke occurrence would fall by 40%. Cardioembolic stroke is caused by an embolism of thrombus from within the heart to a usually normal intracranial artery. Atrial fibrillation is the most common cardioembolic source, and thrombus in the left atrial appendage is the most common source of the thrombus, in chronic or paroxysmal atrial fibrillation. Cardioembolism arises from valvular heart disease, mural thrombus after acute myocardial infarction, paradoxically across an atrial septal defect (possibly including patent foramen ovale), and from intracardiac tumors such as atrial myxoma or fibroelastoma. Large-artery disease does not imply occlusion of a large artery such as the middle cerebral artery. It implies that the source of embolus is an atherosclerotic plaque in the carotid or vertebral or less commonly an intracranial artery. Plaque instability and rupture may occur, analogous to plaque rupture in a coronary artery, and a thrombus will form on the plaque due to exposure of thrombogenic components of the atherosclerotic plaque. An arteroembolus then travels to the brain, blocking a usually normal underlying intracranial vessel. Far less commonly, in situ thrombosis occurs on an intracranial artery plaque. Lacunar or small vessel disease, in contrast, does imply occlusion of a small penetrating artery or arteries in the intracranial circulation. These arteries are too small to visualize adequately on conventional angiography, and so their occlusion is inferred from the small size (< 15 mm) and deep white matter location of the infarct. Lacunar infarcts are caused by (1) intrinsic disease of these small penetrating arteries, such as degenerative lipohyalinosis associated with chronic hypertension or microatheroma associated with atherosclerosis; or (2) embolic occlusion from cardioembolic or arteroembolism from large artery sources. Although intrinsic disease of the small penetrating arteries is more common, it is an error of terminology to associate all lacunar infarcts with intrinsic small-vessel disease. Patients with an acute “lacunar” infarction benefit from thrombolysis, and patients with a carotid artery stenosis and lacunar stroke benefit from carotid endarterectomy.7,8 Finally, there are multiple varied other causes of ischemic stroke. These include blunt arterial injury after trauma, extracranial artery dissection, moyamoya syndrome, infectious causes such as herpes simplex virus or varicella zoster virus–associated vasculitis, bacterial endocarditis, metabolic stroke associated with mitochondrial disease, inflammatory conditions such as Takayasu’s arteritis, and many other conditions. When stroke has been thoroughly investigated and no cause is identified, stroke is labeled as cryptogenic. Naturally, this label must always be considered in light of the full knowledge of what kind of workup was completed. Acute stroke can generally be diagnosed in a straightforward manner; problems in diagnosis arise when the history is incomplete or the examination not thorough. Stroke is sudden and it is statistically the most common cause of an acute neurologic deficit in both young and old. Therefore, an acute neurologic deficit is stroke until proven otherwise. In contrast, stroke type cannot be diagnosed clinically. A stroke syndrome is undifferentiated until imaging defines the distinction between hemorrhagic and ischemic forms. Some clinical features such as sudden headache, altered level of consciousness and signs of mass effect are more commonly associated with acute hemorrhage, but these signs do not have adequate specificity to aid in clinical decision making.9 Stroke can be thought of in four clinical syndromes defined by the Bamford et al.10 These are the total anterior circulation syndrome (TACS), partial anterior circulation syndrome (PACS), lacunar syndrome (LACS), and posterior circulation syndrome (POCS), which roughly define, in order, an M1-MCA occlusion, a branch MCA occlusion, a small penetrating artery occlusion, and a posterior circulation occlusion. The definitions are as follows: (1) TACS entails a triad of hemiparesis (or hemisensory loss), dysphasia (or other new higher cortical dysfunction), and homonymous hemianopia. (2) PACS entails two of the features of TACS, or isolated dysphasia or parietal lobe signs. (3) LACS entails pure motor stroke, pure sensory stroke, sensorimotor stroke, ataxic hemiparesis, or dysarthria-clumsy hand syndrome. (4) POCS entails brainstem or cerebellar signs, or isolated homonymous hemianopia. Stroke can be confirmed with the brain imaging provided by computed tomography (CT) or magnetic resonance imaging (MRI). Alternately in the modern era, stroke is localized to the relevant artery that is occluded. The use of CT angiography in acute stroke and the evolution of endovascular therapy have made arterial localization a more relevant discriminating tool. In the era of acute stroke thrombolysis, decisions about the diagnosis and treatment must be made within a few minutes and most often with incomplete information. Thus, there is much discussion about “stroke mimics” of an acute stroke presentation, and they must be considered carefully in the differential diagnosis (Table 20.1). Imaging of the brain in the acute setting is typically completed with brain noncontrast CT. Attention must be paid to the quality of imaging and technical acquisition parameters of the scanner to optimize the image quality. Acute MRI is an alternate but is less readily available and is disadvantaged in the acute stroke setting by the necessary time required for imaging. CT is insensitive to small volume ischemia, whereas MRI, using echo-planar techniques to produce diffusion-weighted imaging, demonstrates exquisite sensitivity of infarcts smaller than 1 mL.11 As discussed, imaging and its interpretation are critical to acute stroke diagnosis (Table 20.1). Table 20.1 Stroke Mimics Included in the Differential Diagnosis
Ischemic Stroke Diagnosis and Management
What Is a Stroke? How Common Is Stroke? What Are the Major Risk Factors?
Diagnosing Stroke
Clinical Syndromes
Imaging
Common Stroke Mimics | Comments | Pitfalls |
Todd’s paresis | An unwitnessed seizure leaves a patient with focal neurologic signs. | Ischemia can cause an acute seizure. Ensure that the patient does not have an acute arterial occlusion. |
Migraine | Migraine with aura is a neurologic condition that results in brain dysfunction and acute symptoms. | There is very often a history of aura or a family history of migraine with aura. Be sure. Diffusion-weighted magnetic resonance imaging can rule out a stroke. |
Somatization | Somatoform symptoms associated with psychological angst are very common in all cultures and ethnic backgrounds. True malingering or Munchausen syndrome presenting as stroke are rare. | Typically there are inconsistencies on examination. Do not be fooled. Diffusion-weighted magnetic resonance imaging can rule out a stroke. |
Metabolic disturbance/systemic infection | Multiple metabolic conditions (hypoglycemia, drug overdose, hyponatremia, systemic infection, etc.) may present with an acute focal deficit. This is particularly true when there has been previous brain injury, such as a prior stroke. In this case, the patient presents with reemergence of the prior stroke symptoms. | Do not be lulled into missing a basilar artery occlusion by investigating metabolic causes of coma first. Get a picture of the basilar artery with a CT angiogram. |
Subdural hemorrhage | Subdural hemorrhage, particularly acute-on-chronic subdural, may present with sudden focal deficits. | Beware the isodense subdural hemorrhage, as it can be easily missed on CT. |
Glioma | Tumors may similarly present with sudden focal deficits. | Imaging is needed to identify a glioma. |
Dementia | Patients with advanced dementia may present with sudden focal neurologic syndromes. These may have an underlying metabolic cause, but often the cause is not determined. | Obtain a careful history of cognitive status. |
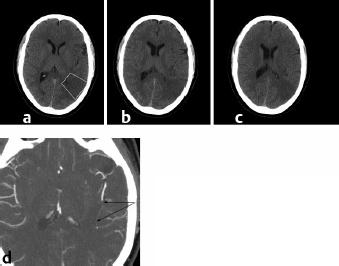
Imaging the arteries is equally critical. CT angiography is emerging as a vitally important tool in the management of acute stroke. Rapid knowledge of the extracranial and intracranial vasculature is highly useful in making diagnostic interpretation and subsequent management decisions (Fig. 20.1).
Treatment
Acute Medical Care
Acute ischemic stroke that is disabling, that presents in a hospital setting, and that can be treated within 4.5 hours of onset should be considered for intravenous thrombolysis with tissue plasminogen activator (tPA) (Alteplase) at 0.9 mg/kg.12 It should be given as a 10% bolus dose (0.09 mg/kg IV push) and then the remaining dose (0.81 mg/kg) infused over 60 minutes.13 The critical issue with stroke thrombolysis is that it must be administered soon after stroke onset. Current guidelines in North America and Europe advise treatment with a door-to-needle time of less than 60 minutes. However, treatment within 30 minutes of arrival is possible and necessary if excellent outcomes are to be achieved.13,14 Acute ischemic stroke treatment with thrombolysis should be administered with exactly the same level of alacrity as an emergency craniotomy.
Acute management of blood pressure and glucose, and issues of general medical support are poorly defined in acute stroke. A major randomized controlled trial is examining the issue of glucose control in acute stroke—the Stroke Hypoglycemia Insulin Network Effort (SHINE) trial (Clinicaltrials.gov number NCT01369069). Blood pressure is managed based on expert opinion; no randomized trials have been done in this area.
Medical therapy subsequently should be conducted on a stroke unit.15 A stroke unit is a dedicated, geographic unit in which stroke patients are cared for by a team of experts. This team-based care is essential to the good outcomes achieved with stroke units compared with general medical wards. The team should consist of a stroke physician, stroke nursing staff, physiotherapists, occupational therapists, speech-language therapists, a social worker, a physiatrist, and others. A team-based approach to care builds expertise. The prevention of stroke-related complications is a critical component of stroke unit care.16 These complications include aspiration pneumonia, venous thromboembolism (deep venous thrombosis [DVT] and pulmonary embolism [PE]), recurrent stroke and progression of stroke, and urinary tract infection. Concurrently, investigations of the stroke mechanism and risk stratification can be done, with the subsequent implementation of stroke prevention treatments, education of patients and families about the condition, and the beginning of stroke rehabilitation. Stroke units reduce hospital length of stay, reduce morbidity and mortality, and improve quality of life.17–20 Stroke units are cost-neutral in most jurisdictions compared with general medical wards.21
Specific Neurosurgical Issues in Acute Stroke Care
Several specific ischemic stroke syndromes require early management and the careful judgment of the neurosurgeon.
Malignant Middle Cerebral Artery Syndrome and Decompressive Hemicraniectomy
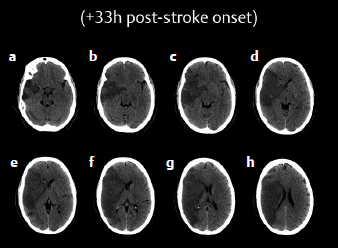
There is strong evidence from randomized clinical trials that decompressive hemicraniectomy with duraplasty is both lifesaving and disability preventing.22 But this evidence pertains only to patients under the age of 60. Despite the randomized trial evidence, many questions remain about patient selection and treatment (Fig. 20.2).
Some issues are clear. Patients with a large MCA infarction should be transferred early to a neurosurgical center for observation and imaging every 12 hours. The procedure should include a wide, large hemicraniectomy, typically with a cruciate dural incision to allow brain swelling. Excision of brain tissue is not necessary or desirable. Careful postoperative nursing is required for the next 4 to 8 weeks until the bone flap can be replaced. The bone flap may be placed in cryostorage in antibiotic solution or may be placed in the peritoneum.
Although the trials found no difference in global outcomes between left and right hemisphere hemicraniectomy, there remains a bias toward performing this procedure for malignant right MCA infarction. It remains unknown if this is reasonable or not. Our experience is that involvement of the MCA plus another territory portends a very poor outcome after this procedure. A carotid “T” occlusion with involvement of the anterior cerebral artery (ACA) territory, or, if there is a fetal-type posterior cerebral artery (PCA), involvement of the occipital lobe, implies greater brain injury and a much poorer trajectory in rehabilitation.
The optimal timing of the procedure is also poorly defined. All patients should have brain imaging completed every 12 hours during the medical management phase. Not all patients who are identified in the acute phase as possibly requiring hemicraniectomy will actually need it. Some can be managed medically; they suffer a period of brain edema and do not herniate and die. Many neurosurgeons are therefore reluctant to operate early, preferring to see evidence of compromise of the intracranial compartment before proceeding to the operating room. In the trials, patients were operated upon within 48 hours of their admission to hospital. In our view an early operative approach is highly desirable. The procedure is generally benign. The infectious risk is low, and postoperative nursing is relatively easily managed. Waiting for signs of herniation is inviting disaster. The procedure is a prophylactic one that should be done early.
Large Inferior Cerebellar Infarction and Decompressive Suboccipital Craniectomy
Occlusion of the fourth segment of the vertebral artery due to atherosclerotic plaque or dissection, or embolic occlusion of the posterior inferior cerebellar artery (PICA), can result in infarction of the inferior cerebellum, with or without concurrent infarction of the inferolateral medulla (Wallenberg syndrome). Where the PICA is dominant, a large infarction may occur. With the evolving vasogenic edema, the patient may suffer malignant infarction with rostral-caudal herniation of the tonsils, medullary compression, and death. The situation is exactly analogous to malignant MCA infarction.
Treatment with early suboccipital decompressive craniectomy may be lifesaving. Similar to the malignant MCA patients, all patients with the potential need for suboccipital craniectomy should be transferred to a neurosurgical center, be managed on a high observation neurologic unit, and be imaged every 12 hours during the medical management phase. Not all patients will require surgery, and observation is warranted according to the imaging. As with the malignant MCA syndrome patient, intervention is best done early with a wide craniectomy and duraplasty. Excision of brain tissue is not required or desirable in most circumstances, except when a brain tissue excision (partial cerebellectomy) is required to obtain adequate decompression.
Patients who herniate rostrocaudally at the foramen magnum die quickly—in a few minutes. There are few warning signs clinically. Again, the intervention is a prophylactic one and is best performed early.
The placement of an external ventricular drain in this syndrome is somewhat risky and certainly controversial. There is the risk of upward herniation of the superior vermis of the cerebellum with compression of the mesencephalon. Further, the general problem is usually not obstructive hydrocephalus but rather focal edema at the site of infarction. Overall, our belief is that if there is a consideration of placing an external ventricular drain (EVD), then it is probably time to deal with the problem definitively and perform a suboccipital decompressive craniectomy.
Endovascular Treatment
Endovascular treatment for acute ischemic stroke is based on the principle that restoration of blood flow is the best way to improve outcome. The technology has rapidly developed and evolved over the past decade. Initial work with delivery of thrombolytic drugs at the site of the thrombus evolved to the use of a guidewire and microcatheter for mechanical disruption of thrombus.23–26 Subsequent innovation has led to the mechanical thrombectomy procedure with the Concentric Merci retriever (Concentric Medical, Mountain View, CA) and now a new class of stentriever devices.27–31 In parallel, aspiration thrombectomy has been developed using the Penumbra Stroke System (Penumbra Inc., Alameda, CA)32 or simply a large-bore guide catheter and a 60-cc syringe.
Three recent trials have failed to demonstrate that endovascular treatment is superior to standard intravenous thrombolysis.33–35 These trials have cast a pall on the field but have rekindled equipoise regarding the role of endovascular treatment.36 Currently, some private insurers have withdrawn coverage for this procedure. But in truth these trials are only the opening foray. There were three key weaknesses to these trials: (1) suboptimal devices were used, with subsequent incomplete revascularization; (2) treatment was too slow; and (3) optimal patients for this therapy were not chosen using modern imaging techniques. New trials are being designed to assess the patient population, and it seems likely that the population to be treated will be further defined with new trials.
Endovascular treatment for vertebral artery or carotid artery sacrifice is rarely encountered in ischemic stroke syndromes. Rarely, a vertebral artery dissection will be unstable and present with recurrent thromboembolism. Coil occlusion and vessel sacrifice may be required to prevent major stroke. Similarly, pseudoaneurysm formation after dissection may be managed safely with vessel sacrifice.
Open Embolectomy
There are case reports of open craniectomy with microvascular intracranial embolectomy.37,38 However, with the evolution of endovascular treatments for acute ischemic stroke that are faster and less invasive, this treatment is no longer widely used. If it is to be performed in an exceptional circumstance, such as a foreign body embolism, it must be done quickly to obtain a reasonable outcome, and it requires a highly technically adept surgeon to complete the procedure in a timely fashion.
Carotid Revascularization
Optimal stroke prevention commonly requires carotid revascularization. Carotid endarterectomy (CEA) is the preferred approach for the uncomplicated patient. It is associated with a clear reduction in stroke or death as compared with carotid artery stenting.39–42 Among patients with poor anatomy (high bifurcation, isolated circulation), carotid artery stenting may be preferred. Carotid artery stenting may also be ideal for patients who are judged to be at high risk for a general anesthetic; however, among these high-risk patients, the reflective surgeon should carefully judge whether carotid intervention should be performed at all.
A key issue is that carotid revascularization must be done relatively quickly. The benefit of surgery fades quickly after 2 weeks have elapsed since the stroke event.43 Thus, the surgeon needs to be quick about triaging referrals and getting operative intervention organized and safely completed. Whether it is safe to operate early or later in this 2-week window remains unclear and is an urgent question to be resolved by a large-scale randomized clinical trial.44
Stroke After Carotid Endarterectomy or Stenting
Stroke after carotid endarterectomy is fortunately uncommon. Over the course of two decades of CEA studies, the rate has fallen below 2%.45 Perioperative stroke is more common after carotid artery stenting.45 Mechanisms of stroke after CEA include thrombosis at the operative site with carotid occlusion, arteroembolism, or both. Prolonged clamp times in the setting of inadequate collateral circulation may result in stroke without obvious occlusion. Other mechanisms of stroke may coexist, such as atrial fibrillation, which can result in cardioembolic events occurring perioperatively.
A rapid clinical diagnosis and arterial diagnosis must be achieved. CT angiography is very helpful acutely. Reoperation with thrombus aspiration is a possible acute treatment. Endovascular intervention may be undertaken for distal arteroembolic occlusion. In the setting of partially occlusive thrombus at the site of the arteriotomy, we have found that intravenous bolus dosing of platelet antagonists such as abciximab is useful.46 It has been demonstrated that acute loading of clopidogrel reduces emboli detected by transcranial Doppler ultrasonography.47,48
Direct Extracranial–Intracranial Bypass
Direct extracranial (EC) to intracranial (IC) vessel bypass is rarely used in acute stroke. It is reserved for unusual situations such as moyamoya syndrome.49 Rarely, young and otherwise healthy patients might have bilateral carotid dissections or other massive impairment of the proximal or intracranial circulation. Such patients may be candidates for direct EC–IC bypass to restore the circulation and prevent a slow, evolving infarction. Individualized patient selection, guided by imaging, is required to identify these uncommon patients. Decisions to proceed with this kind of intervention will never be guided by randomized controlled trials but instead by clinical evaluation, physiology, surgical expertise, and imaging.
Blunt Arterial Injury and Dissection in the Trauma Patient
The blunt trauma patient may have suffered enough force to the neck to result in blunt arterial injury. This is common in the vertebral arteries when there is a fracture extending through the transverse foramen in the lateral process of the vertebrae, indicating substantial force application in this anatomic region. Carotid artery injuries may also occur, typically at the skull base. Although orthopedically stable, these injuries may result in stroke due to arteroembolism of an intraluminal thrombus at the site of injury. Sometimes there is a frank dissection of the artery; however, it is critical to label these injuries as blunt arterial injuries, with awareness that one subset of these is traumatic arterial dissection. Treatment is typically medical with antiplatelet (acetylsalicylic acid [ASA], clopidogrel) or antithrombotic medication (heparins). The prognosis is typically good; only a minority of patients will suffer a stroke.
Screening of trauma patients with CT angiography identifies many patients with asymptomatic dissection. Without pseudoaneurysm formation, these lesions tend to be benign and can be managed expectantly and conservatively just with ASA daily. Patients with an SAH associated with a dissecting pseudoaneurysm are at high risk of death. Management usually required sacrifice of the relevant artery.
Pharmacologic Treatment with Fibrinolytic and Antithrombotic Agents
Fibrinolytic Agents
In North America, tPA is the most used fibrinolytic agent for stroke both intravenously and endovascularly. Streptokinase trials were done early, and streptokinase was associated with an increased risk of intracerebral hemorrhage. Tenecteplase (TNK-tPA, TNKase) is under ongoing investigation for stroke and may replace tPA in the future.50–52 Desmoteplase is similarly under ongoing investigation for stroke.53 Urokinase is still available in some jurisdictions and has been used both intravenously and intra-arterially with success.
Antiplatelet Agents
Acetylsalicylic acid (ASA, aspirin) is the most commonly used antiplatelet agent. It inhibits cyclooxygenase-1 enzyme, reducing platelet aggregation. It is safe and inexpensive, and modestly beneficial in preventing stroke. Clopidogrel is a prodrug that is metabolized in the liver to an active thiol metabolite, which irreversibly inhibits the platelet P2Y12 adenosine diphosphate receptor, blocking platelet aggregation. Clopidogrel requires oral administration, and a loading dose is required to obtain adequately rapid platelet inhibition. The novel drugs ticagrelor and prasugrel, also P2Y12 adenosine diphosphate receptor inhibitors, have not been tested in stroke, but both are promising because they are available in intravenous formulation.
The combination of ASA and clopidogrel has been shown to reduce the chance of early recurrent stroke after initial TIA or minor stroke.54,55 Similarly, treatment with clopidogrel after CEA has been shown to reduce postoperative emboli detected by transcranial Doppler.47
The glycoprotein (GP) IIb/IIIa inhibitors abciximab and eptifibatide have been investigated in stroke. Abciximab was not associated with a better outcome in routine acute ischemic stroke treatment. Eptifibatide is being investigated in combination with low-dose tPA for the treatment of acute ischemic stroke. These drugs are used in the endovascular suite when patients suffer thrombotic complications of aneurysm coiling or other intra-arterial interventions. However, no studies that have convincingly demonstrated that this is an effective and safe approach to treatment. Experience suggests that intra-arterial administration of abciximab is associated with recanalization of occluded intracranial arteries after iatrogenic occlusion with aneurysm coiling. Tirofiban, a nonpeptide GPIIb/IIIa antagonist, reduces cerebral embolism from carotid artery plaque.56 Routinely, these drugs are not used in the treatment of stroke.
Anticoagulants
In general, both unfractionated and low molecular weight heparins are not beneficial in ischemic stroke. There is a small increase in the risk of major intracranial hemorrhage. Trials that have examined heparin use are now dated; patients with stroke enrolled in these trials were not differentiated with imaging. We reserve the use of heparins for situations where there is proven intravascular thrombus, limit its use, and use conservative approaches such that the partial thromboplastin time (PTT) or anti-Xa activity is not excessive at first dosing. The risk of major hemorrhage with unfractionated heparin is, in part, related to longer PTT times. In general, very few patients with ischemic stroke warrant treatment with full-dose heparins.
Pulmonary thromboembolism (DVT and PE) prophylaxis warrants the use of heparin. We used enoxaparin 40 mg once daily given subcutaneously based upon the PREVAIL study.57
Oral Anticoagulants and Reversal
Oral anticoagulation using a vitamin K antagonist (coumarin) is the accepted standard for stroke prevention in atrial fibrillation and for other potential cardioembolic sources of stroke (e.g., prosthetic heart valve). Recently, novel oral anticoagulants have been introduced. These include dabigatran, a direct thrombin inhibitor, and rivaroxaban, apixaban, and edoxaban, which are direct factor Xa inhibitors. Dabigatran, rivaroxaban, and apixaban are now approved in most jurisdictions for stroke prevention in atrial fibrillation. A similar path is expected for edoxaban.
For the neurosurgeon, a key issue with these medications is reversal when there is a need for urgent surgery. Vitamin K antagonists may be reversed with prothrombin complex concentrates (Octaplex™, Beriplex™) and vitamin K and fresh frozen plasma. Prothrombin complex concentrates work in minutes. Fresh frozen plasma takes hours to infuse and reestablish normal clotting parameters. Vitamin K restoration results in a normal international normalized ratio (INR) in 12 to 24 hours when the liver has normal synthetic function. Factor VIIa administration will normalize the INR, but it is less clear if clotting is truly normalized.
In contrast, there are no rapidly acting reversal agents for any of the novel anticoagulants. Because the half-life of these drugs is on the order of 8 to 12 hours, fully normal coagulation will not occur for 2 to 3 days with normal metabolism. Some blood products, including activated prothrombin complex concentrates (e.g., factor eight inhibitor bypass activity [FEIBA]) or activated factor VIIa combined with tranexamic acid have been suggested.
• Time is brain in most aspects of acute neurosurgical care of stroke. Care delayed is care denied.
• Decompressive hemicraniectomy and suboccipital craniectomy are lifesaving procedures that are ideally performed early in the course of stroke.
• Carotid revascularization is not a procedure that can wait. In good operative candidates, it should be performed within days of the symptomatic event.
• Carotid endarterectomy is the preferred procedure, over carotid artery stenting, for stroke prevention.
• The management of the acute ischemic stroke patient is a team affair. The vascular neurosurgeon should work closely with the stroke unit team to ensure effective and rapid care can be delivered.
• The vascular neurosurgeon plays a key role on the stroke team.
• Routine concerns such as carotid revascularization play a day-to-day role.
• Less commonly the surgeon has a critical role to play in managing severely affected patients with malignant MCA or PICA territory infarction.
• The surgeon-interventionalist may play a critical role in the endovascular management of acute ischemic stroke.