Limbic Anatomy and Physiology
Dan C. McIntyre
Philip A. Schwartzkroin
Introduction
The concept of a “limbic lobe” was first put forth by Broca27 as a means of labeling the various structures that surround the brainstem and form the border of the ventricular system. Broca was responsible also for the view of these structures as primarily olfactory in function (a major input from olfactory lobes)—i.e., as a “smell brain” (rhinencephalon). Based on anatomic grounds, Papez182 proposed the existence of a network of limbic structures—including the hypothalamus, anterior thalamus, cingulate cortex, and hippocampus—that was responsible for emotional behavior. The amygdala was not included in the initial description of the Papez circuit, but Maclean139 subsequently expanded Papez’s view of the anatomy of emotion to include the amygdala and several parahippocampal structures; he called this expanded network the limbic system. Although both Papez and Maclean viewed the hippocampus as central to the limbic system concept of emotion, we now know that emotional behavior is more strongly supported by the amygdala.151,201,271 In contrast, the central position of the hippocampus in the limbic system has more recently focused on its role in memory61,228,229,247 and its contributions to temporal lobe epilepsy (TLE).74
Of the various epileptic disorders seen in humans, the most frequently observed are those of temporal-lobe origin.62 Considering the pathogenesis of the temporal lobe seizures, one often (but not always) finds a sclerotic lesion in one or more of the limbic or mesial temporal structures (see Chapter 13). These mesial structures form part of an olfactory–neocortical network that involves the hippocampus; the entorhinal, perirhinal, and piriform cortices; and the amygdala. The cells contained within these structures have both intrinsic properties and local connections that, when sufficiently provoked, can provide strong recurrent excitation that leads to robust seizure activity. The strong communication between these structures can amplify the seizure event and recruit cells with efferent connections that distribute widely throughout the brain. It is of little surprise, therefore, that discrete electrical stimulation in several of these same mesial structures can reproduce, in an epileptic patient, many of the features of the patient’s automatism,259 and that surgical resection of the structures can provide relief from the seizures.63 With this evidence in mind, we briefly review both anatomic and physiologic features of the hippocampus, entorhinal cortex, perirhinal and piriform cortices, and amygdala. A description of the normal structure and function of these “limbic”/mesial temporal structures is critical for our understanding of their roles in the development, study,126 and expression of TLE.73
The Hippocampal Formation
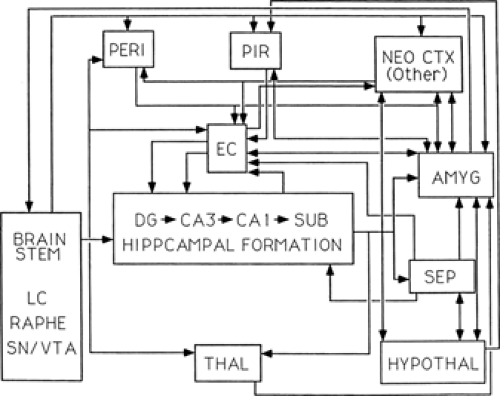
The hippocampal formation has attracted considerable attention during the recent explosion of interest in cellular and synaptic neuroscience.215,218 Its attraction as a focus for research stems from several considerations: (a) It is a region of the brain implicated in a number of important (and interesting) “normal” behaviors, such as learning and memory; (b) both functionally and structurally, the hippocampus shows an unusual degree of neuronal “plasticity”129,172,214; (c) it has been implicated as a focus of pathology in a number of neurologic disorders ranging from epilepsy to global ischemia,24 Alzheimer disease,161 traumatic head injury,261 to psychiatric disorders38; (d) its unique, laminated structure has made it particularly conducive to study using in vitro brain slice preparations, an approach that has been adopted by many laboratories; (e) it can be viewed as a somewhat simplified cortex; and (f) it is richly connected to other parts of the limbic system (Fig. 1). The archicortical hippocampal circuitry has served as a “model” for the more complex neocortex, and the hippocampal pyramidal cell has been studied and characterized as a model central nervous system (CNS) neuron. Given the interest and activity of so many investigators, a large store of information is available about hippocampal cell properties, structure, afferents and efferents, receptors and transmitters, and local circuits. This chapter deals primarily with major points of organization and function that have potential implications for our understanding of epilepsy-related phenomena.
Regional Connectivity
The hippocampal formation is conventionally divided into four major cell regions: Subiculum, cornu ammonis regio superior (CA1), cornu ammonis regio inferior (CA3), and the dentate gyrus (Fig. 1). Each region is defined, at least in part, by its unique patterns of input and output, as well as by the features of its principal cell population. Part of the beauty and simplicity of the hippocampus is in the early established finding that each region projects to the next through an excitatory “trisynaptic” pathway7 (see Fig. 1). Thus, the granule cells of the dentate gyrus send their mossy fiber axons to the CA3 region (the CA2 transition zone, by definition, receives no mossy fiber input); axon branches of the CA3 pyramidal cells—the Schaffer collaterals—project to CA1; the CA1 pyramidal cells send axons to the subiculum; and the subiculum projects out of the hippocampal formation (back to entorhinal cortex as well as other cortical targets). Recent studies have suggested that this simple view of an intrahippocampal organization is unrealistic7 and perhaps even misleading in our efforts to learn how information is processed through the hippocampus. We now know, for example, (a) that the mossy fibers also make numerous contacts within the dentate hilus and that they colocalize opioid peptides152 and zinc,68 along with glutamate; (b) that the CA3 cells project to contralateral hippocampus, as well as back into the ipsilateral dentate hilus, where they excite mossy cells and interneurons65,127; (c) that activity in the CA1 pyramidal region can influence activity in the CA3
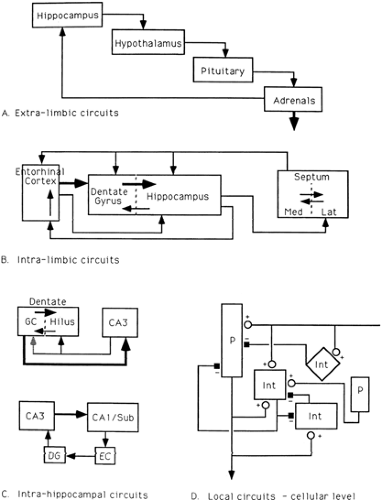
pyramidal cells, perhaps through antidromic mechanisms233; (d) and that hilar neurons interconnect ipsilateral and contralateral dentate regions.200 Further, although it appears that each of the major associational connections uses the excitatory neurotransmitter, glutamate, the nature of the evoked post-synaptic potentials in each region may be rather different. For example, the mossy fiber unitary excitatory postsynaptic potential (EPSP) onto CA3 pyramidal cells is very large (millivolts) and mediated primarily by non-N-methyl-D-aspartate (NMDA) glutamate receptors270; in contrast, the unitary EPSP produced by Schaffer collateral synapses onto CA1 pyramidal cells is more typical in size (about 100 μV) and involves both non-NMDA and NMDA receptor components.204 Finally, hippocampal “interneurons” contribute a γ-aminobutyric acid (GABA)ergic component to interregional, as well as local intraregional, information relay.219 Thus, what was once thought to be a simple, serial, feed-forward intrahippocampal pathway is now known to be an elaborate series of interacting and parallel feed-forward and feedback circuits, involving not only principal cell axons but also specialized interneuronal interactions (Fig. 2).
pyramidal cells, perhaps through antidromic mechanisms233; (d) and that hilar neurons interconnect ipsilateral and contralateral dentate regions.200 Further, although it appears that each of the major associational connections uses the excitatory neurotransmitter, glutamate, the nature of the evoked post-synaptic potentials in each region may be rather different. For example, the mossy fiber unitary excitatory postsynaptic potential (EPSP) onto CA3 pyramidal cells is very large (millivolts) and mediated primarily by non-N-methyl-D-aspartate (NMDA) glutamate receptors270; in contrast, the unitary EPSP produced by Schaffer collateral synapses onto CA1 pyramidal cells is more typical in size (about 100 μV) and involves both non-NMDA and NMDA receptor components.204 Finally, hippocampal “interneurons” contribute a γ-aminobutyric acid (GABA)ergic component to interregional, as well as local intraregional, information relay.219 Thus, what was once thought to be a simple, serial, feed-forward intrahippocampal pathway is now known to be an elaborate series of interacting and parallel feed-forward and feedback circuits, involving not only principal cell axons but also specialized interneuronal interactions (Fig. 2).
Afferents (Inputs)
Cortical Afferents
The major cortical input to the hippocampal formation arises in layer II pyramidal cells of the entorhinal cortex (EC), projects
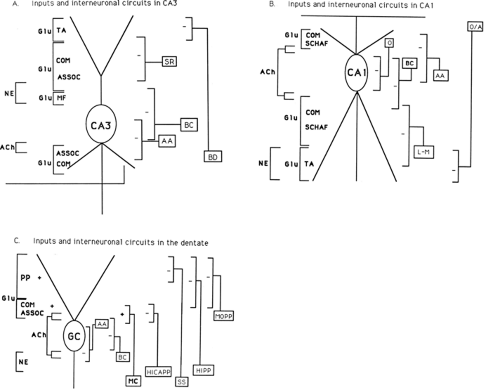
through the angular bundle pathway, and enters the ipsilateral dentate gyrus via the perforant pathway.94,235 This excitatory input terminates largely on spines on the distal dendrites of granule cells (and on interneurons) in the outer two thirds of the dentate molecular layer (Fig. 3). The entorhinal input has been subdivided into two divisions, with fibers originating in the medial EC synapsing in the middle third of the molecular layer and fibers from the lateral EC synapsing in the outer third. Investigators continue to explore differential features of these subdivisions, some of which may be relevant to dentate seizure susceptibility. For example, the medial division is glutamatergic and evokes EPSPs with both NMDA and non-NMDA components; in the lateral division, opioid-dependent long-term potentiation26 is supported by anatomically defined colocalization of glutamate and enkephalin, with the latter “transmitter” affecting regional interneurons (inhibition via μ- and δ-receptors) to mediate disinhibition of granule cells269—a perhaps critical step in dentate seizure genesis.
through the angular bundle pathway, and enters the ipsilateral dentate gyrus via the perforant pathway.94,235 This excitatory input terminates largely on spines on the distal dendrites of granule cells (and on interneurons) in the outer two thirds of the dentate molecular layer (Fig. 3). The entorhinal input has been subdivided into two divisions, with fibers originating in the medial EC synapsing in the middle third of the molecular layer and fibers from the lateral EC synapsing in the outer third. Investigators continue to explore differential features of these subdivisions, some of which may be relevant to dentate seizure susceptibility. For example, the medial division is glutamatergic and evokes EPSPs with both NMDA and non-NMDA components; in the lateral division, opioid-dependent long-term potentiation26 is supported by anatomically defined colocalization of glutamate and enkephalin, with the latter “transmitter” affecting regional interneurons (inhibition via μ- and δ-receptors) to mediate disinhibition of granule cells269—a perhaps critical step in dentate seizure genesis.
Anatomic studies of the EC projection to the hippocampal formation have also identified an input to hippocampus proper.
This temporoammonic pathway arises in layer III cells of EC but separates from the perforant pathway to contact the most distal branches of pyramidal cells in the stratum lacunosum-moleculare of CA1 to CA3.264 Although clearly excitatory (i.e., glutamatergic), the functional nature of this pathway has been questioned because its influence on pyramidal cell discharge has been difficult to demonstrate.225 Recent experiments suggest that temporoammonic modulation of pyramidal cells is significant, and that this input may also differentially activate an interneuron subpopulation located in these distal reaches of the apical dendritic region (see Fig. 3).123 It is worthwhile to note that the direction of input to cornu ammonis via this entorhinal input is opposite to that of the associational input via the major trisynaptic pathways; thus, pyramidal cell excitation and processing cannot be viewed as a one-way serial influence. The function of this pathway remains unclear but appears to provide a frequency-dependent modulatory action from the entorhinal cortex directly onto hippocampal pyramidal cells.107
This temporoammonic pathway arises in layer III cells of EC but separates from the perforant pathway to contact the most distal branches of pyramidal cells in the stratum lacunosum-moleculare of CA1 to CA3.264 Although clearly excitatory (i.e., glutamatergic), the functional nature of this pathway has been questioned because its influence on pyramidal cell discharge has been difficult to demonstrate.225 Recent experiments suggest that temporoammonic modulation of pyramidal cells is significant, and that this input may also differentially activate an interneuron subpopulation located in these distal reaches of the apical dendritic region (see Fig. 3).123 It is worthwhile to note that the direction of input to cornu ammonis via this entorhinal input is opposite to that of the associational input via the major trisynaptic pathways; thus, pyramidal cell excitation and processing cannot be viewed as a one-way serial influence. The function of this pathway remains unclear but appears to provide a frequency-dependent modulatory action from the entorhinal cortex directly onto hippocampal pyramidal cells.107
Subcortical Afferents
A variety of subcortical fiber inputs innervate the hippocampal formation, some in very specific laminar patterns and others in a more diffuse pattern (see Fig. 3). The major subcortical projections include:
The septohippocampal input arises in the medial septal nucleus and diagonal band of Broca and enters the hippocampus via the fimbria/fornix.268 This input provides the hippocampal formation with the vast majority of its acetylcholine (ACh)131 and comprises a subcomponent of the basal forebrain cholinergic projection to cortex that has been implicated in learning and cognitive function. However, it is now very clear that this input consists of both cholinergic and GABAergic components6; septohippocampal lesion experiments, designed to deprive hippocampus of its ACh supply, must be carefully interpreted in light of damage to both GABAergic and cholinergic inputs. Acetylcholinesterase staining, which disappears almost entirely with septal lesions or with fimbria/fornix transection, shows a discrete pattern of staining in all regions of hippocampus, with a concentration in thin supra- and subcellular regions (Fig. 3). Interestingly, recent studies suggest that some interneuron subpopulations are extremely sensitive to ACh197 and may be excited at much lower thresholds than are the principal cells. This possibility is intriguing, inasmuch as at least one form of hippocampal θ-activity—the slow, rhythmic EEG activity that is associated with restful alertness—is mediated via cholinergic mechanisms128 and may well depend on excitation of interneurons for synchronization of the hippocampal projection cell populations.237 The cholinergic synaptic action in hippocampus is primarily muscarinic (blocked by atropine). The GABAergic projection from septum to hippocampus appears to have a slightly different set of targets. Although it is difficult to identify the general pattern of septal GABA fiber ramification in hippocampus (because of the large intrinsic GABA network), tracing studies have shown that septohippocampal GABA fibers preferentially synapse on GABAergic basket cells in the dentate gyrus,69 forming a pathway for hippocampal/dentate disinhibition.
The brainstem monoamine pathways also provide an important, presumably modulatory, influence on hippocampal neurons. Noradrenergic fibers arising from the locus coeruleus,135 serotonergic fibers arising from the raphe nuclei,170 and dopaminergic fibers (although very sparse) from brainstem nuclei have been shown to ramify diffusely within all regions of hippocampus. The noradrenergic input, although generally diffuse, is most obvious in the CA3 and dentate hilar regions. However, cells in all parts of hippocampus have been shown to be sensitive to norepinephrine (NE), primarily (but not exclusively) via a β-adrenergic receptor mechanism217 that leads (via cyclic adenosine monophosphate [cAMP] production) to a blockade of a hyperpolarizing potential (with loss of spike-firing adaptation) and net cell excitation.140 Norepinephrine has been implicated in a form of long-term potentiation (LTP) in the CA3 pyramidal cell region,97 as well as in the dentate.232 How these noradrenergic effects on hippocampal cells are related to whole-brain hyperexcitability is unclear because lesions of the ascending noradrenergic system yield a nervous system with heightened seizure susceptibility.153 A mouse “knockout” of the norepinephrine-synthesizing enzyme (dopamine β-hydroxylase) also shows increased seizure susceptibility.244 In contrast to this net excitatory effect of NE in hippocampus, both serotonin 5-hydroxytryptamine (5-HT) and dopamine (DA) produce hyperpolarizing (inhibitory) responses in hippocampal pyramidal cells, mediated by G-protein–coupled mechanisms.9,22 The role played by these potent modulators of cell excitability is yet to be well characterized. However, serotonergic fiber input, like the septal GABAergic input, has been recently traced to a specific contact with interneurons within the dentate hilus87; specific serotonergic effects on interneurons may well be critical in the net influence of this pathway in hippocampus. Clearly, the effects of these monoaminergic afferents in hippocampus depend on the postsynaptic receptor subtypes upon which they synapse.10 Interneurons exhibit 5-HT3 receptors, which are colocalized with CB1 cannabinoid receptors, thus suggesting not only a specific serotonin modulation of interneurons, but also a complex interaction between 5-HT and the endocannabinoid system.171
Efferents (Outputs)
Cortical Projections.
The major hippocampal cortical efferents arise in the subiculum and CA1 regions and project to the lower layers of entorhinal cortex.265 This system completes a feedback loop to the EC (through hippocampus) (see Fig. 2), because the deep cortical layers of EC then influence the output of layers II and III projection cells.5 In addition, subicular fibers project to the perirhinal cortex, another component of the “parahippocampal” complex. Anatomic and electrophysiologic studies show that CA1 and subiculum send axons to widespread prefrontal cortices, including the prelimbic area, cingulate cortex, medial orbital cortex, and the infralimbic areas.102,180,258 Although the roles of these efferent projections have not yet been clearly understood, these connections are consistent with a role for parahippocampal and prefrontal cortices in cognitive processes. Further, because medial/orbital prefrontal regions have brainstem connections that modulate autonomic function, these hippocampal connections to prefrontal cortex may endow the hippocampus with a role in emotional and visceral aspects of behavior long associated with the limbic region.
Subcortical Projections.
The best studied of the subcortical hippocampal projections are the “reciprocal” connections from CA1 and subiculum back to the septum—but to the lateral, rather than medial, septal nucleus.265 Again, because the lateral septal nucleus connects closely with the medial septal nucleus (from which hippocampal afferents arise), this projection constitutes part of still another feedback loop between the hippocampus and a related structure (see Fig. 2). The nucleus accumbens of the basal forebrain is a related projection from the same general subregions of subiculum. This structure is associated with the “limbic” component of the ventral basal ganglia. The subiculum also provides important connections from the hippocampal formation to the thalamus (midline nuclei), amygdala, and hypothalamus (ventromedial nucleus and mamillary body).37,93 Although these projections “make sense” in terms of the old Papez circuit view of limbic system involvement in emotional and visceral behaviors, the actual role of these efferents (and of the reciprocal connections
to hippocampus, largely through septum) are still to be determined. One additional circuit of note includes the hippocampus as the component of the hypothalamic-pituitary-adrenal (HPA) axis.76 Adrenal steroid receptor (glucocorticoid and, especially, mineralocorticoid) concentration is high in the hippocampus, and its modulation of hippocampal output may play a key role in controlling the release of corticotropin-releasing hormone (CRH) and in corticotropin release from hypothalamus (i.e., in feedback control of adrenal steroid secretion). The hippocampal efferents to hypothalamus may thus be critically involved in adrenal stress responses.253
to hippocampus, largely through septum) are still to be determined. One additional circuit of note includes the hippocampus as the component of the hypothalamic-pituitary-adrenal (HPA) axis.76 Adrenal steroid receptor (glucocorticoid and, especially, mineralocorticoid) concentration is high in the hippocampus, and its modulation of hippocampal output may play a key role in controlling the release of corticotropin-releasing hormone (CRH) and in corticotropin release from hypothalamus (i.e., in feedback control of adrenal steroid secretion). The hippocampal efferents to hypothalamus may thus be critically involved in adrenal stress responses.253
Regional Characteristics
Dentate Gyrus.
The dentate gyrus is the primary target of cortical input to the hippocampal formation and consists of the granule cell region and the related dentate hilus. The granule cell region is neatly laminated,23 with the granule cell bodies densely packed in stratum granulosum (SG) and their dendrites reaching through the stratum moleculare (SM) (see Fig. 3). The stratum moleculare is conventionally divided into thirds: The inner third is the focus of associational, commissural, and septal input; the middle third receives input from the medial EC; and the outer third receives input from the lateral EC. Partially surrounded by stratum granulosum is the hilus, home to a variety of polymorphic cells that include the excitatory (glutamatergic) mossy cells (primary source of the dentate commissural and associational connections) and inhibitory interneurons.4 Until recently, the dentate region of hippocampus was relatively neglected by epileptologists because it appeared to have a very high threshold for seizure activity.138 Recent studies, however, have focused more closely on this region because (a) the granule cells are relatively resistant to seizure-associated damage224; (b) granule cell axons show a high degree of plasticity (sprouting) in epileptic brain42; (c) changes in synaptic currents and voltage-dependent channels have been demonstrated in granule cells following kindling165; and (d) maximal activation of this region is associated with the generalization of seizure activity through the limbic system and beyond.138 Indeed, the dentate is now sometimes considered the gatekeeper of hippocampal excitability.91
Granule cells are unusual because they show constant turnover throughout life, regulated in part by adrenal steroids and stress,75 exercise,112 and even seizures.183 The role of newborn granule cells has been discussed with respect to behavioral function,132 hippocampal repair,117 and development of the epileptic state.209 Electrical properties—intrinsic and synaptic—have been extensively studied,211,227,252 and granule cells have been found to be rather unexcitable because they maintain a very negative resting potential. Prolonged depolarization also fails to evoke rapid, repetitive firing for long periods because these cells exhibit pronounced spike firing adaptation; bursts of action potentials are followed by a large after-hyperpolarization. Activation of EC afferents to the dentate evokes a monosynaptic EPSP in granule cells; it is unclear how much of this synaptic excitation in normal hippocampus is mediated by NMDA receptors.165 In addition, this afferent input activates a powerful inhibitory local circuit that rapidly curtails granule cell firing.138 As elsewhere in hippocampus, at least some of the dentate interneurons are activated at a much lower threshold than are the granule cells30; activation of inhibitory interneurons dampens the granule cell discharge so that “physiologic” levels of EC input are likely to result in rather discrete and restricted dentate (i.e., granule cell spiking) output to the hilus and CA3. The granule cell axons (mossy fibers) excite hilar and CA3 neurons (both principal cells and interneurons) via glutamatergic mechanisms.206 These cells colocalize the opioid peptide dynorphin255 and contain a high concentration of zinc68 in their terminals. Although the role of these colocalized substances remains controversial, evidence suggests that zinc may modulate synaptic plasticity (long-term potentiation [LTP]) at the postsynaptic cell133; decrease glutamate release from the presynaptic terminal,12 and even affect GABA receptors in epileptogenic circuits.49,169 Further, it has recently been shown that granule cells/mossy fibers also contain and release GABA—an observation that has obvious implications for “excitatory” neurotransmission in the adult hippocampus and also for hippocampal development in the immature brain.80
In addition to the granule cells, the other “projection” cell type in the dentate is the hilar mossy cell, which sends its axons into the inner SM, both ipsi- and contralaterally, to make excitatory connections on granule cell dendrites. These cells receive their primary input from granule cells (another feedback circuit) (see Fig. 2), although some mossy cells have dendrites that reach into the SM to receive EC input directly.205 Interestingly, mossy cells are among the most vulnerable cell types in the hippocampal formation, and their death is thought to trigger the granule cell axon sprouting response into the inner SM, often seen in epileptic hippocampus.42 It has also been hypothesized that mossy cell damage, by removing a tonic source of excitation to inhibitory interneurons, gives rise to disinhibition of the granule cells.222 Recordings from mossy cells show them to receive a constant “spontaneous” stream of large, non-NMDA EPSPs206 from granule cell mossy fiber terminals that require minimal summation to trigger action potential discharge; thus, it is not surprising that mossy cells have a very low threshold for discharge and may fire in bursts of action potentials when the summed EPSP is very large.32 Recent studies have shown that mossy cells also receive input from CA3 projecting back into the hilus.207 Finally, it is of interest that these cells have none of the calcium binding proteins common in other hippocampal cell types (granule cells contain calbindin). It is thought that inadequate intracellular calcium buffering may contribute to mossy cell vulnerability, a possibility supported by the finding that the injection of an intracellular chelator will save these neurons from potentially toxic levels of excitation210 and the apparent resistance to injury of calretinin-containing mossy cells in the mouse and the gerbil.71,118
Local circuits in the dentate, once thought to be restricted to the relatively simple feedback loop between inhibitory basket cells and granule cells, are shown to be very complex (see Fig. 3). In addition to five subtypes of dentate basket cells,199 the dentate contains a myriad of other GABAergic interneurons. Each subpopulation appears to have a specific dendritic and axonal arborization pattern,89 and some colocalize (with GABA) peptide neurotransmitters. For example, the somatostatin-containing interneurons in the hilus send their axons into the outer molecular layer to interact with distal granule cell dendrites and with the incoming lateral perforant path fibers.130 Although the role played by these interneurons is not yet clear, their mode of termination (and data regarding mechanism of action of somatostatin) suggests that they may have a presynaptic inhibitory function in relation to the perforant path input. Chandelier cells, a unique interneuron cell type, make exclusive—and apparently powerful—inhibitory synapses on the axon hillock and initial segment of hippocampal cells.33,226 Other inhibitory cell types have been described with specific axon terminal fields in the middle or inner SM,89 and one interneuron cell type has now been found that projects to CA3 and CA1.31 Interestingly, all these interneurons appear to contain GABA; no excitatory “interneurons” have been described in the dentate (or anywhere else in hippocampus).
It is clear that each cell type has a special function, as determined by the arborization pattern of its dendritic tree, the localization of its axon terminals, and its colocalized transmitter content. However, because most interneurons synapse with each other, it is often difficult to predict what the net effect of activation of a given subpopulation might be; interneuron
synapses directly onto granule cells may have a clear inhibitory outcome, but interneuron inhibition of interneurons may result in granule cell disinhibition or net excitation. Recent studies have revealed that at least one interneuron population in the dentate appears to be particularly sensitive to GABAB receptor-mediated inhibition164; it has been hypothesized that GABAB receptors on the terminals of GABA interneurons may, in fact, regulate the cell’s release of GABA through a direct inhibitory effect by GABA on its own terminal.173 Different interneurons, too, are differentially sensitive to excitotoxic damage; basket cells (at least those types that contain the calcium-binding protein parvalbumin) are resistant to damage,223 and are well preserved in epileptic hippocampus (even hippocampi showing severe mesial temporal sclerosis), whereas hilar somatostatin-containing interneurons are quite vulnerable (they lack calcium-binding proteins), and their loss may contribute to the hyperexcitability of dentate exposed to high levels of prolonged stimulation.52 Not surprisingly, the different subpopulations of interneurons express different receptor and channel combinations.41 Finally, in interneurons, as in other cells of hippocampus, activity-dependent (seizure-inducing) changes occur in cell properties (e.g., receptor complement, channels.186 For example, neuropeptide Y (NPY) and associated receptors are dramatically regulated by seizure activity46,254; these changes, plus the observation that exogenously modified NPY expression may modify seizure activity, has led to the view that the NPY system constitutes an adaptive feedback system for regulating excitability.254
synapses directly onto granule cells may have a clear inhibitory outcome, but interneuron inhibition of interneurons may result in granule cell disinhibition or net excitation. Recent studies have revealed that at least one interneuron population in the dentate appears to be particularly sensitive to GABAB receptor-mediated inhibition164; it has been hypothesized that GABAB receptors on the terminals of GABA interneurons may, in fact, regulate the cell’s release of GABA through a direct inhibitory effect by GABA on its own terminal.173 Different interneurons, too, are differentially sensitive to excitotoxic damage; basket cells (at least those types that contain the calcium-binding protein parvalbumin) are resistant to damage,223 and are well preserved in epileptic hippocampus (even hippocampi showing severe mesial temporal sclerosis), whereas hilar somatostatin-containing interneurons are quite vulnerable (they lack calcium-binding proteins), and their loss may contribute to the hyperexcitability of dentate exposed to high levels of prolonged stimulation.52 Not surprisingly, the different subpopulations of interneurons express different receptor and channel combinations.41 Finally, in interneurons, as in other cells of hippocampus, activity-dependent (seizure-inducing) changes occur in cell properties (e.g., receptor complement, channels.186 For example, neuropeptide Y (NPY) and associated receptors are dramatically regulated by seizure activity46,254; these changes, plus the observation that exogenously modified NPY expression may modify seizure activity, has led to the view that the NPY system constitutes an adaptive feedback system for regulating excitability.254
CA3.
The CA3 region is composed of large pyramidal cells (and associated interneurons) and is the recipient of mossy-fiber input from the granule cells. The region is conventionally divided into at least three divisions: CA3a (which we shall consider together with CA2 for the purposes of this discussion) is that part of the cell band most distal from the dentate (and closest to CA1); CA3b comprises the middle part of the band, nearest the fimbria/fornix connection; and CA3c is most proximal to the dentate, inserting into the hilus.5 Although each of these divisions may have slightly different characteristics, the region as a whole (including CA2) has been considered to be the “pacemaker” area of the hippocampus.267 In CA3 much of the rhythmic, synchronous bursting activity associated with interictal epileptiform activity appears to be generated. This population property is a product of the intrinsic properties of CA3 pyramidal cells and their high degree of excitatory collateral connectivity.249 Unlike other regions of the hippocampus, CA3 pyramidal cell axon collaterals ramify extensively within the local region and make excitatory contacts with their neighbors. The unique properties of CA3 local circuitry have been implicated in a number of hypotheses regarding learning and memory processes in the hippocampus,81 and also appear to provide a basis for oscillatory rhythms that are characteristic of hippocampus. The CA3 region (as well as entorhinal cortex) constitutes a generator of γ-frequency (10–30 Hz) oscillations, a cholinergically driven pattern that depends on coupling of interneurons (and pyramidal cell axons) via gap junctions, as well as via more conventional chemical excitatory (glutamatergic) and inhibitory (GABAergic) synapses.146,250 The CA3 region also generates sharp waves and high-frequency oscillations—EEG patterns implicated in memory consolidation.142
Although the excitatory output of CA3 is often damped by strong inhibitory interneuronal influences, even minor reductions in inhibitory efficacy in CA3 can result in significant synchronized bursting, due to interaction among CA3 pyramidal cells. This bursting (or oscillatory drive) is relayed to the CA1 region ipsilaterally (via Schaffer collaterals) and to CA1 and CA3 regions contralaterally (the commissural fibers of hippocampus proper). Although this output is effective in driving postsynaptic targets, it also appears to be important in inhibiting the generation of ictal activities, particularly in entorhinal cortex.14 Because of the very extensive nature of the CA3 axon collateralization over the longitudinal extent of hippocampus,134 there is significant divergence of this CA3 output (it is not laminar). Perhaps curiously, this same CA3 region is only reluctantly recruited into ictal-like seizure activity—perhaps because of the strength of its inhibitory circuitry or perhaps because the mechanism of the interictal-like bursts directly antagonizes seizure genesis.104
The intrinsic and synaptic properties of CA3 pyramidal cells determine this unique set of epilepsy-related characteristics. Individual pyramidal cells in this region have an intrinsic burst propensity, apparently based on a relatively high density of calcium channels in their proximal dendrites.66,266 Membrane depolarization (e.g., from incoming synaptic activity) not only may trigger conventional sodium action potentials, but also may open these calcium channels; the calcium influx causes a more prolonged depolarization of the cell, driving additional action potentials in a “burst.” Because afferent input often involves activation of both excitatory and inhibitory influences onto CA3 cells, this burst propensity is generally curtailed by the hyperpolarizing effect of the inhibition.163 When these bursts occur, however, they provide a potent drive, not only to CA1 targets, but also to neighboring CA3 cells (via the excitatory collateral system); a gradual recruitment of CA3 neuron activity can thus lead to synchronized burst discharge. Importantly, there also appears to be a very effective mechanism for turning off these bursts—the after-hyperpolarization generated by a calcium-dependent potassium conductance.98 Thus, the very mechanism of burst generation—calcium influx—also involves a self-limiting process (the calcium-activated hyperpolarization). These processes presumably contribute to the reluctance of CA3 to participate in ictal-like activity, which requires prolonged depolarization and repetitive action potential discharge.
Presumably, the major trigger for CA3 discharge is afferent input from the dentate granule cells. Large mossy fiber terminals engage in very complex synapses on the proximal part of the CA3 apical dendrite in the stratum lucidum, where they contact complex dendritic spines; glutamate release from a single terminal evokes a large non–NMDA-mediated EPSP.28 Fortunately, the baseline “spontaneous” level of granule cell activity is relatively low, so that CA3 cells are not constantly driven at high rates. The unique features of mossy fiber input appear to account for many of the region-specific properties of CA3. Mossy fibers colocalize glutamate with high concentrations of zinc (see earlier discussion), dynorphin, and GABA. Further, the mossy fiber terminals appear to have receptors for kainate (see next section), as well as for BDNF. BDNF application to the CA3 region results in synchronized burst discharge activity.208
Fortunately, the same mossy fiber input that activates CA3 pyramidal cells also drives local interneurons very effectively, so that CA3 cells are tonically inhibited by a variety of interneuron subtypes79 (see Fig. 3). The subpopulations of interneurons in CA3 overlaps with, but is not exactly the same as, those in the dentate; basket cells provide potent inhibition to the level of the cell soma, and other cell types show unique dendritic arborization patterns and region-specific targeting by axon collateral. Investigators have shown that different morphologically defined interneurons also show different electrophysiologic properties; the interneurons include both fast-spiking cells—whose inhibitory postsynaptic potentials (IPSPs) summate to produce small, smooth IPSPs in pyramidal cells—and slow-spiking cells, which produce large, fast-rising IPSPs in the pyramidal cell target.162 As in the dentate, the dendritic region of CA3 is laminated; the most proximal apical dendrite receives mossy fibers exclusively, the mid-dendritic regions (strata radiatum on the apical side and oriens on the basal side) receive primarily associational and commissural fibers (i.e., from other CA3 cells),
and the distal apical dendrites (stratum lacunosum-moleculare) receive input from the temporoammonic afferents (from EC) (Fig. 3). All these excitatory glutamatergic inputs are modulated not only by the local GABAergic circuits but also by the subcortical afferent systems. Interestingly, the mossy fiber input to CA3, because it is mediated by non-NMDA glutamate receptors, exhibits a different form of synaptic plasticity256 from the typical LTP (or long-term depression [LTD]) so intensively studied as a cellular model of learning (and a possible contributor to kindling). The plasticity of this synapse, in contrast to the LTP produced in stratum radiatum of the same region, appears to be particularly dependent on (sensitive to) monoaminergic activation of a cAMP second-messenger system.97 Recent studies suggest that interneurons specific to the stratum lucidum are key participants in mossy fiber–induced synaptic plasticity.184
and the distal apical dendrites (stratum lacunosum-moleculare) receive input from the temporoammonic afferents (from EC) (Fig. 3). All these excitatory glutamatergic inputs are modulated not only by the local GABAergic circuits but also by the subcortical afferent systems. Interestingly, the mossy fiber input to CA3, because it is mediated by non-NMDA glutamate receptors, exhibits a different form of synaptic plasticity256 from the typical LTP (or long-term depression [LTD]) so intensively studied as a cellular model of learning (and a possible contributor to kindling). The plasticity of this synapse, in contrast to the LTP produced in stratum radiatum of the same region, appears to be particularly dependent on (sensitive to) monoaminergic activation of a cAMP second-messenger system.97 Recent studies suggest that interneurons specific to the stratum lucidum are key participants in mossy fiber–induced synaptic plasticity.184
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree