Fig. 23.1
Sagital T1-weighted cranial magnetic resonance (MR) imaging showing the hyperintense appearance of a small curvilinear lipoma of the interhemispheric fissure
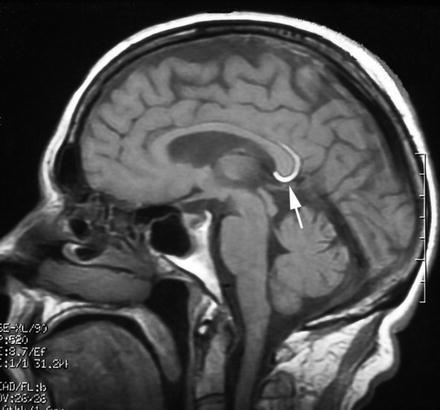
Fig. 23.2
(a) Axial T1-weighted cranial magnetic resonance (MR) imaging showing the hyperintense appearance of a giant interhemispheric lipoma; (b) Axial MR T2-weighted images showing the same lesion with reduced density; (c) Coronal MR fat saturation pulse sequence of the same lipoma, agenesis of the corpus callosum can be appreciated
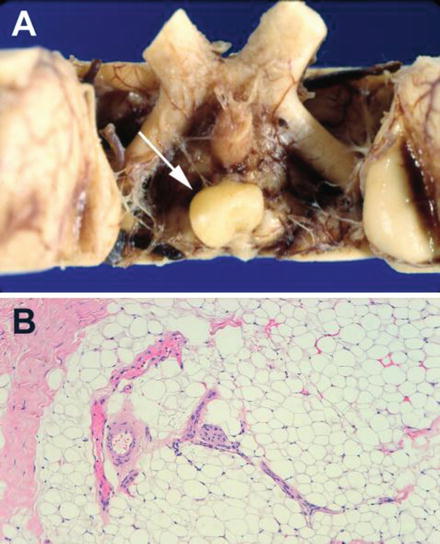
Fig. 23.3
(a) Macroscopic appearance of a small lipoma of the suprasellar region attached to the hypothalamus. (b) Photomicrograph of a lipoma composed of mature adipose cells
Epidemiology
Intracranial lipomas are usually silent and this fact explains the difficulty of a proper statistical assessment, therefore no robust data concerning the prevalence of intracranial lipomas are available. The first descriptions of intracranial lipomas were achieved mainly through incidental findings at autopsy (Jeffers et al. 2009). The expanded use of neuroimaging techniques has allowed increasing the diagnosis of lipomas. Intracranial lipoma accounts for 0.46–1 % of all intracranial tumors (Donati et al. 1992). Prevalence of intracranial lipomas detected on cranial MRI in the patient population of a hospital was 0.045 % (17/38,000) (Kemmling et al. 2008). Spinal intradural lipoma is a rare condition and occurs in approximately 1 % of all primary spinal cord tumors (Vila Mengual et al. 2009). Lipomas of the spinal cord are frequently associated with spina bifida and are more commonly located in lumbosacral region. The thoracic spinal cord is the second most common region of involvement followed by the cervicothoracic and cervical regions. Intradural lipomas of the spinal cord with intracranial extension are very rare (Şanh et al. 2010).
Pathogenesis
Truwit and Barkowich (1990) have reviewed the numerous theories regarding the pathogenesis of intracranial lipomas. The precise etiopathology of intracranial lipomas has been a topic of discussion. Theories regarding the hystogenesis of these lesions include: (1) hypertrophy from the pre-existing fatty tissue of the meninges, (2) metaplasia of meningeal connective tissue, (3) heterotopic malformation of dermal origin, (4) tumor like malformation derived from the primitive meninx and (5) fatty degeneration of proliferated glia. Today intracranial lipomas are accepted to be the result of meningeal maldifferentiation and they are genetically linked to other midline defects due to the improper closure of the neural tube. The presence of lipomas is explained by the abnormal persistence of the meninx primitive, a mesenchymal derivative of the neural crest that is usually reabsorbed in an orderly fashion during embryogenesis giving rise to the subarachnoid cisterns. When the meninx primitive does not regress into subarachnoid space and maldifferentiates into adipose tissue a lipoma originates. This theory explains the cisternal locations of lipomas and the intralesional locations of blood vessels and cranial nerves.
The malformative mechanism of lipomas is supported by its association with midline malformations of the cns in up to 40 % of cases (Gómez-Gonsálvez et al. 2003). They are mainly associated with maldevelopment of the corpus callosum in the form of agenesis/dysgenesis. Other anomalies of the cns associated with lipomas are the absence of septum pellucidum, cranium bifidum, spina bifida, encephalocele, myelomeningocele and hypoplasia of cerebellar vermis.
There are also reports of cortical abnormalities associated with cerebral lipomas such as architectural disorganization and focal penetration of fibroadipose tissue into the brain parenchyma. As the formation of a lipoma takes part of a complex malformation that involves sulcus formation and cortical development within its vicinity, it may interfere with the growing of cortical tissue during the ongoing formation of the sylvian fissure, resulting in cortical dysplasia of the vicinity (Kakita et al. 2005).
In rare occasions intracranial lipoma is associated with subcutaneous lipoma. In this case different communication patterns can be observed between intracranial and extracranial component of the lipoma. They may have no connection (Tubbs et al. 2007), may connect to each other by a fibrous lipomatous stalk (Yamashita et al. 2005) or may have direct continuity with each other through cranium bifidum (Sethi et al. 2008). A case of intraextracranial lipoma associated with sagittal sinus fenestration, absent straight sinus and falcine sinus has been described.
The development of a lipoma may also involve vascular abnormalities and a variety of vascular abnormalities have been described in association with intracranial lipoma including dilatation, tortuosity or narrowing of feedings arteries and veins, engulfment of the cerebral arteries, arteriovenous malformations and aneurysms and malformations of venous sinus (Saatci et al. 2000). Hypervascularization has been observed adjacent to and within a lipoma located in the Sylvian fissure (Kakita et al. 2005).
Two groups of interhemispheric lipomas have been described (Yildiz et al. 2006). Tubulonodular type is characterized by nodular lesions smaller than 2 cm. They are a result of a more severe insult that occurs at an early embryonic stage and interferes with the normal development of the corpus callosum (Fig. 23.1). It is located anteriorly with the epicentre in the genu in 83 % of cases and associated with a high incidence of cranial defects, frontal masses and encephaloceles. Curvilinear lipomas are thin and located posteriorly around the splenium (Fig. 23.2). This type is generally associated with a normal corpus callosum and has a low incidence of associated anomalies.
Pathology
Macroscopically lipomas vary in size from subcentimeter nodules to large masses. They have a bright yellow appearance and a soft, lobulated and fibrous tissue consistency not easily fragmentably. Microscopic examination after hematoxylin and eosin staining reveals mature adipose tissue surrounded by a fibrous capsule with varied amounts of collagen and blood vessels (Fig. 23.3). The capsule and surrounding parenchyma frequently contain calcifications (Feldman et al. 2001). An exceptional myelomatous change in a lipoma has been described by Suri et al. (2008). These authors consider that metaplastic differentiation in the lipoma gave origin to myelolipoma which contain hematopoietic elements including erytrocytes, myeloid cells, megakaryocytes and focal lymphoid aggregate formation.
Clinical Manifestations
Although the prevalence of symptomatic lipomas remains controversial, epilepsy, headache, psychomotor retardation and cranial nerve paralysis may occur (Venkatesh et al. 2003; Yilmaz et al. 2006). Most intracranial lipomas are considered to be asymptomatic and frequently they are an incidental finding in patients undergoing a cranial computerized tomography (CT) or magnetic resonance (MR) after a cranial trauma (Lin et al. 2009).
Epilepsy is the most common symptom associated with lipomas. A 5 % of cranial lipomas present with epilepsy (Gómez-Gonsálvez et al. 2003). Epileptic seizures have been noted in a large proportion of patients with sylvian lipoma presumably due to irritation of the mesiotemporal cortex or to the variety of associated neocortical abnormalities (Saatci et al. 2000; Feldman et al. 2001; Vela-Yebra et al. 2002; Yildiz et al. 2006). However the association of interhemispheric lipomas and epilepsy remains controversial (Martínez-Lapiscina et al. 2010). Some authors have suggested that the symptomatic nature of corpus callosum lipomas is dependent on the interruption of the callosal fibers, which are replaced by the neoplasm. Such disconnection is responsible for each hemisphere to develop epileptic discharges. However, few case reports reported clinical and electroencephalographic characteristics congruent with cranial MR or CT which could allow attributing the etiology of epilepsy to the lipoma. Loddenkemper et al. (2006) reviewed 3,500 epilepsy patients for the presence of intracranial lipomas; only five cases were found and epilepsy could be linked to the lipoma in only a single patient. Therefore, the authors suggested that intracranial lipomas were incidental findings in this population. Yilmaz et al. (2006) reported a prevalence of epilepsy of 20 % in an adult case series and Gómez-Gonsálvez et al. (2003) reported a prevalence of 5 % in a similar pediatric case series.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






