♦ Preoperative
Imaging
- Use magnetic resonance imaging as a screening tool to determine possible levels of involvement
- Standing lumbar radiographs with flexion and extension view to assess segmental motion
- Computed tomography or bone scan if integrity of pars is questioned
- Provocative lumbar discography for concordant confirmation of disease
Choice of Arthroplasty Device
Charité
- U.S. Food and Drug Administration (FDA) approved for single level from L4–S1
- Biarticulating mobile core with variable center of rotation
- Metal-on-polyethylene: two cobalt-chrome endplates and an ultra-high molecular weight polyethylene core
- Spike fixation to endplates
PRODISC-L
- FDA approved for single level from L3–S1
- Ball-and-socket configuration with fixed center of rotation
- Metal-on-polyethylene: two cobalt-chromium endplates and an ultra-high molecular weight polyethylene core
- Center keel fixation to endplates
Maverick
- Awaiting FDA approval for single level from L4–S1
- Ball-and-socket configuration with fixed center of rotation
- Metal-on-metal: cobalt-chromium-molybdenum
- Center keel fixation to endplates
Patient Counseling
- Discuss risks, potential benefits, and possible complications
- Discuss motion-sparing technology versus fusion
- Make patient aware of risk of adjacent segment disease
- Inform patient of radiographic imaging difficulties with arthroplasty
Operating Room Set-up
- Intraoperative fluoroscopy
Anesthetic Issues
- Steroids: dexamethasone 10 mg intravenously given prior to incision
- Antibiotics: cefazolin 2 g intravenously given prior to incision
- Anticoagulation: enoxaparin 30 mg subcutaneously prior to incision for levels above L5–S1
- General endotracheal anesthesia with muscle relaxant
♦ Intraoperative
Positioning and Exposure
- The patient is placed in a supine position or in the French position with the legs spread (with the surgeon standing between the patient legs for direct anterior access to the lumbar spine).
- The lumbar spine may be approached through either a transperitoneal or retroperitoneal exposure. The amount of great vessel release and retraction should be limited to that required for insertion of the instruments and constructs.
- At L4–L5, the iliolumbar and segmental vessels should be identified and ligated to facilitate mobilization of the vena cava.
- At L5–S1, the middle sacral artery and vein are ligated and divided. Minimize cautery use along the anterior aspect of the spine to avoid injury to the presacral sympathetic plexus.
- A lateral fluoroscopic image is used to confirm the targeted lumbar level.
- An anteroposterior fluoroscopic image with Ferguson projection is used to identify midline.
- Mark the centerline location of the vertebral bodies above and below the operative level (with cautery, a chisel, or with a center-marking pin for the Maverick).
Discectomy and Endplate Preparation
- Identify and mark the lateral margins of the discectomy.
- Incise the annulus sharply.
- Use a Cobb elevator to elevate the cartilaginous endplate.
- Use the pituitary rongeurs to remove the nucleus pulposus.
- The discectomy should clear the disc space all the way to the posterior ligament.
- Endplates may be prepared using a high-speed burr or decorticated with a ring curette.
Disc – Space Distraction
- Using blunt-nose scrapers, release any ligamentous adhesions to allow the operative level to move naturally.
- Up-and-down-angled Kerrison rongeurs may be used to remove osteophytes from the posterior margin of the disc.
- Tension the disc spreader while fluoroscopically watching the motion of the vertebrae to evaluate whether the operative level is moving properly.
Sizing and Placement of the Artificial Disc
PRODISC – L (Fig. 118.1)
- Insert trial implant to determine proper size and positioning.
- If trial implant moves away from the midline, remove it and perform additional mobilization and distraction of the disc space.
< div class='tao-gold-member'>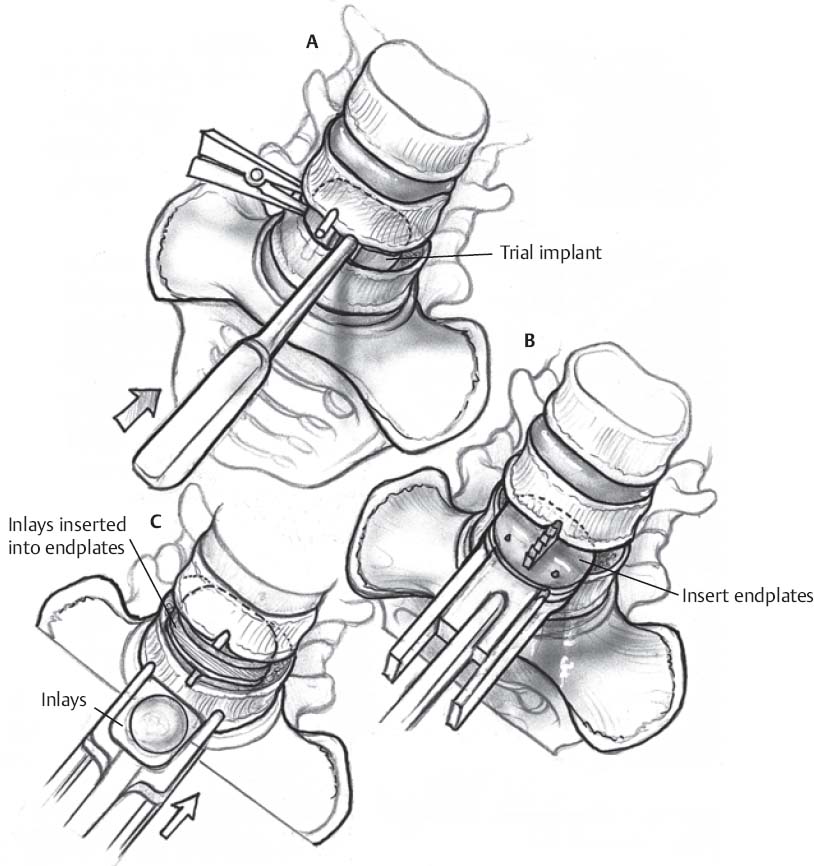 Only gold members can continue reading. Log In or Register to continue
Only gold members can continue reading. Log In or Register to continue







