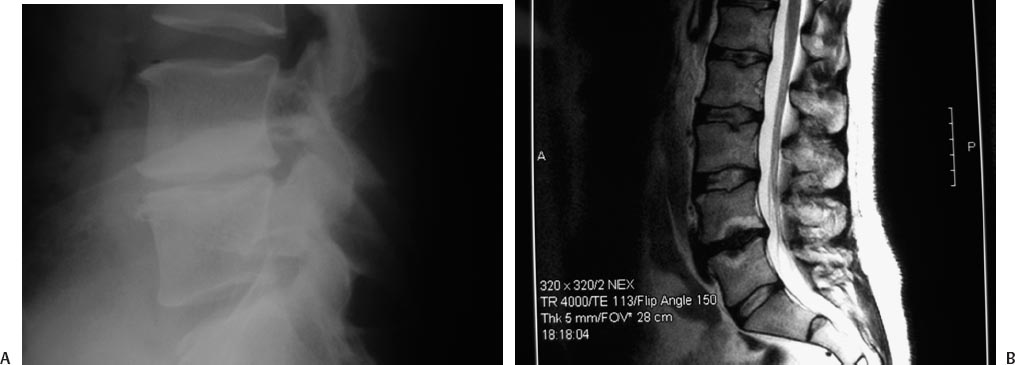
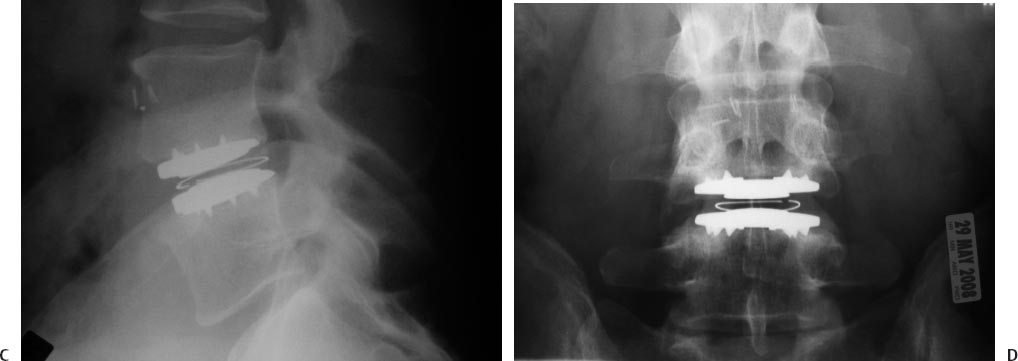
25 Lumbar Spine Arthroplasty Charles W. Davis and Paul C. McAfee Spinal fusion was traditionally the surgical solution to disabling arthritic conditions where stiffness and loss of function were acceptable trade-offs for relief of disabling pain. To date, most spine surgery consists of “salvage” procedures (e.g., correcting the effects of trauma, stabilizing and correcting deformity, fusing degenerative segments) and, similarly, does not address restoration of normal biomechanical function. In fact, although lumbar fusion is still the treatment of choice and the gold standard for many severe spinal disorders, it has been shown to restrict motion and increase stress on adjacent levels, which years later, could result in additional pain and surgery.1,2 Posterolateral lumbar fusion was described in the 1930s and was shortly thereafter followed by anterior lumbar interbody fusion (ALIF) in 1933, posterior lumbar interbody fusion in 1944, pedicles screw augmentation in the 1980s, and recombinant human bone morphogenetic protein (rhBMP-2) in 2003. Even with all these technical and scientific advancements, the average clinical success and fusion rates after fusion surgery still only reach 75% and 85%, respectively. This finding was presented in a recent review by Bono and Lee involving 4,454 patients from 78 reports over 20 years. The authors also reported that the length of time to fusion was 3 to 24 months. They hypothesized that fusion could cause adverse effects at adjacent levels and further discussed the complexity of surgical treatment for low back pain (LBP).3,4 Despite these less-than-perfect clinical outcomes, however, Fritzell et al5 prospectively demonstrated that compared with the nonoperative care, lumbar spinal fusion significantly improved outcomes in patients with chronic LBP. Unlike fusion, lumbar total disc replacement (TDR) is intended to maintain motion at the spinal segment, restore proper disc height, and maintain segmental lordosis. Compared with arthrodesis, disc replacement presents two key theoretical advantages: (1) the risk for pseudarthrosis, a possible complication of fusion, is eliminated; and (2) by maintaining motion at the index level, adjacent segment disease may be minimized. The effectiveness of arthroplasty devices to maintain motion was shown in both preclinical and clinical studies; several cadaveric studies demonstrated that artificial discs normalized disc motion.1,6 Furthermore, clinical studies also confirmed restoration of radiographic motion.7–9 Thus, although no long-term study has yet been published on the protective impact of arthroplasty on adjacent-level degeneration, early evidence has demonstrated some level of motion preservation at implanted levels. The first disc replacement, developed by Hamby and Glaser in 1959, was an acrylic mass to be injected into an evacuated disc space after total discectomy. It showed no obvious clinical advantage and was soon replaced by an injectable silicone insert developed by Nachemson in 1962. This device, however, performed poorly in mechanical testing and did not proceed to clinical application. The first clinical series of disc replacements was performed by Dr. Fernstrom in the early 1960s; he evaluated a stainless steel ball bearing put in the disc space following discectomy.10 After a short period of pain relief, the device predictably subsided into the vertebral body. It took another 20 years for the next-generation device to enter the realm of clinical trials. The SB Charité I was first developed in 1984 by Drs. Schellnack and Büttner-Janz. It consisted of nonforged stainless steel and contained no special instrumentation.11 This device was quickly followed by the Charité II in 1985 and then by the third generation in 1987, consisting of cast cobalt chrome endplates and an ultra- high-molecular-weight polyethylene (UHMWPE) sliding core. First released in 1987, discs were implanted worldwide and data on the clinical performance of the device became available soon thereafter.12 The Charité artificial disc was then evaluated in the United States in a randomized controlled trial (RCT) against fusion with anteriorly placed stand-alone BAK cages and iliac crest autograft,8,13 and obtained approval for market release in 2004. In addition to the Charité artificial disc (DePuy Spine, Raynham, MA), other arthroplasty devices have recently been developed and are entering the market. Specifically, ProDisc-L (Synthes, Inc., West Chester, PA) was granted Food and Drug Administration (FDA) approval in 2006 and described in a recent peer-reviewed publication.14 The Maverick artificial disc (Medtronic Sofamor Danek, Memphis, TN), Kineflex lumbar disc (SpinalMotion, Inc., Mountain View, CA), and FlexiCore lumbar discs (Stryker Spine, Kalamazoo, MI) completed their randomized enrollments and are currently in continued access nonrandomized modes. The Kineflex lumbar disc is the only lumbar disc, thus far, to be randomized against another disc (CHARITÉ) in FDA trials. All these ongoing and completed randomized clinical trials have generated a large body of evidence on the efficacy and safety of arthroplasty for lumbar spine. Understanding the biomechanics of healthy intervertebral discs (IVDs) is critical when evaluating new devices for spinal arthroplasty. A functional spine unit consists of two vertebral bodies and the intervening IVD. Included in this unit are also a pair of posterior facet joints and the associated complex of ligaments, tendons, and muscles. The IVD is avascular and consists of the anulus fibrosus, mostly made of type I collagen and the nucleus pulposus, made of type II collagen primarily. The nucleus consists of 88% water as well as proteoglycans (i.e., glycosaminoglycans). As for the anulus fibrosis, it presents a radial arrangement of type I collagen fibers that overlap in 90-degree biased layers. These layers resist distension and torsion forces. The IVD thus allows motion and absorbs compression forces. Uniform internal distribution of load and pressure can thus be found in a healthy disc. Much like larger joints such as the knee, the motion in a healthy lumbar disc is characterized by a dynamic center of rotation (COR).6,15 This moving core enables independent translation and rotation, which are key components of physiologic motion. With age, however, the disc tends to degenerate and respond with less elasticity and less uniform pressure distribution. Two principal biomechanical designs are currently advocated by various device manufacturers: (1) to replicate this moving COR within the artificial disc, or (2) to replace the diseased disc with an artificial disc with a fixed COR. The theory behind the mobile core is that it attempts to maintain the motion of the operative spinal segment in a fashion similar to that of an intact nucleus. It has also been shown in biomechanical studies to decrease the load on the facets in flexion.16 Replicating this moving COR has therefore been a key engineering focus for some of the lumbar arthroplasty devices, such as the Charité artificial disc. As for the fixed core design, it represents the “averaging” for instant access of rotation. This design is subject to less shear, which may protect the facets. It is, however, less forgiving to malpositioning as the COR of the device is fixed. The biggest challenge with lumbar arthroplasty is not technical, but rather one of patient selection (Fig. 25.1). Arthroplasty is primarily used to treat discogenic pain. The cause of discogenic pain is not uniformly and conclusively known; however, recent studies indicate that production of proinflammatory mediators within the nucleus pulposus of degenerated discs may be causing the pain. Indeed, when comparing the levels of interleukin-6 (IL-6), interleukin-8 (IL-8), and prostaglandin E2 in explanted disc tissues from sciatica patients (i.e., no disc pain) versus fusion patients (i.e., high disc pain), a statistically significant difference in the production of IL-6 and IL-8 was observed between the sciatica and fusion groups.17 Fig. 25.1 Indications for lumbar arthroplasty: a typical case example. (A) A 47-year-old white male executive who has been disabled for 9 months due to lumbar spondylosis at L4-L5, mechanical back pain radiating down the posterolateral aspect of his right thigh with an Oswestry Disability Index of 55. (B) His sagittal MRI scan discloses single-level disc disease at L4-L5, Modic type II changes. His discogram was also positive for concordant pain when his L4-L5 disc was stimulated, but not when L3-L4 or when L5-S1 were injected. He underwent in motion lumbar disc arthroplasty (the updated version of the Charité) with complete relief of his mechanical back pain, ODI = 5 postoperatively, and returned to work fulltime. (C) Postarthroplasty lateral radiograph, which demonstrates good restoration of disc space height, uniformly successful with many types of arthroplasty. (D)The anteroposterior radiograph with the in-motion Charité arthroplasty shows midline ideal position of this nonkeeled type of lumbar disc replacement. The difference between a painful and an asymptomatic disc may therefore only be biochemical in nature and two radiographically identical “black discs” may elicit a completely different physiological response. In fact, up to 30% of all black discs are asymptomatic. This is why diagnosis of discogenic pain is challenging as imaging technologies such as magnetic resonance imaging (MRI) and computed tomography (CT) do not have the necessary specificity to precisely localize pain generators.18 In the early 1990s, analysis of the high-intensity zone (HIZ) on MRI was initially met with much enthusiasm as it reported sensitivity of 86% compared with discograms.19 This enthusiasm, however, was soon mitigated by subsequent reports, devaluating the sensitivity of HIZ to much lower levels (27 to 31% and up to 52%) and further describing limitations of this technique, which also led to false-positives.20,21 The key conclusion here is that surgeons must not fall prey to treating diagnostic results and must correlate the patients’ clinical presentation with diagnostic studies to arrive at an outcome-oriented treatment plan. The best diagnostic approach to try and localize back pain, therefore, must include radiographic methodologies, such as CT and MRI, as well as pain-specific diagnostic tools, such as discograms or nerve block injections, to provide a clear connection between the radiographic dark disc and the actual source of pain. Indications for use for the currently available lumbar arthroplasty devices (Charité artificial disc and ProDisc-L total disc replacement) are very similar. As an example, the Charité artificial disc is indicated for spinal arthroplasty in skeletally mature patients with degenerative disc disease (DDD) at one level from L4-SI, and ProDisc from L3-S1. These patients should have no more than 3 mm of spondylolisthesis at the involved level. Patients should have failed at least 6 months of conservative treatment prior to implantation of the prosthesis. Symptoms related to the degenerative segment can include chronic LBP with or without leg pain. Furthermore, foraminal stenosis secondary to disc space height loss may be relieved indirectly by disc height restoration. The contraindications are numerous and include active systemic infection or infection localized to the site of implantation, osteoporosis and osteopenia, bony lumbar stenosis, allergy or sensitivity to implant materials, pars defect, isolated radicular compression syndromes especially due to disc herniation, scoliosis with greater than 11 degrees of coronal deformity, instability (isthmic spondylolysis or retro or anterolisthesis >3 mm), central stenosis, tumor, advanced facet disease, and poor psychometric evaluation. Approach-related contraindications include anterior vascular calcification, previous major vessel surgery, a body mass index above 40, and previous retroperitoneal procedures.8,13,22 As recently described, the surgical approach for TDR is similar to that for an ALIF.23 The patient is supine on a radiolucent table. Arms should be positioned to not interfere with the lateral x-ray. An anterior retroperitoneal approach is preferred with a longitudinal incision lateral to the linea alba. Once the disc and posterior longitudinal ligament are excised and endplates are prepared, there are several key issues that the surgeon needs to understand to promote a successful outcome. First, the importance of sizing cannot be overemphasized. The implant must maximize coverage of the vertebral endplate. The endplate center and anterior perimeters are weaker than the posterolateral and periphery areas. Proper sizing therefore reduces the potential for subsidence. Care must be taken to avoid pushing any disc material into the spinal canal during placement of the prosthesis. Midline placement has also been shown to correlate with improved clinical outcomes. Most manufacturers have modular components, which allow for the surgeon to use lordotic endplates and minimize shear. The most lordotic endplate must then be placed inferiorly to make the joint plane more horizontal. This, in turn, neutralizes the forward displacement tendency and reduces any excessive shear load, unloading the facet joints. Complications from spinal arthroplasty have been well documented, for both marketed products (such as the Charité artificial disc and the ProDisc-L) as well as devices in development (the Maverick total disc arthroplasty system). Most complications requiring revision surgery were resolved by either fusion and/or disc replacement surgery. The revisability of spinal arthroplasty devices has been thoroughly described in the published literature.24 Beyond the general complication rates described in the main randomized controlled trials for Charité and the ProDisc-L publications, other investigators have reported various rates and causes of complications. Tropiano et al reported a 9% complication rate and a 6% revision rate in 53 patients treated with the ProDisc-L with an average follow up of 1.4 years.25 Complications included vertebral body fracture, transient radicular pain, implant malposition, and transient retrograde ejaculation. The issue of vertebral body fracture, a complication specific to devices with keels, was also recently described by Shim et al26 in two cases that were not revised or treated surgically, but experienced prolonged back pain. An additional complication in the form of acquired spondylolysis was recently described by Schulte et al.27 Device failure and polyethylene wear debris, complications specific to devices containing UHMWPE cores, were presented by Punt et al and Van Ooij et al, both describing the same patient population.28,29 It is worth noting that earlier devices were susceptible to this type of wear because of suboptimal sterilization processes. Nowadays, devices are sterilized in conditions that protect their biomechanical integrity.30 Neurologic complications were also evaluated within the RCT patient population that made up the Charité investigational device exemption (IDE) study. From these data, Geisler et al31 concluded that the rate of neurologic complication was “exceedingly low” in both, the fusion and arthroplasty groups, with no statistically significant differences between groups. Metallosis and metal ion release from metallic arthroplasty devices have also been investigated as potential complications. An early case of device removal describing severe metallosis was recently published.32 Zeh et al33 further discussed release of cobalt and chromium ions from the artificial disc into the bloodstream. Authors described the concentrations of Cr/Co measured in the arthroplasty patients as “similar in terms of their level to the values measured in metal-on-metal THA [total hip replacement] combinations or exceed those values reported in the literature.” Long-term clinical studies will thus be needed to further confirm the safety of these devices. Despite all the above-mentioned possible complications, the most commonly reported failure is facet joint arthrosis. Facet degeneration is a contraindication for arthroplasty. In long-term studies, David and Lemaire et al described 5 cases (4.7%) and 11 cases (11%) of facet arthrosis at the latest time point (10-year postoperatively), respectively.34,35 In other studies, facet degeneration was shown as high as 30% at 3-year follow-up. It is unclear, however, whether patients included in this 3-year study had completely healthy facet joints at time of surgery, or whether some level of degeneration had already occurred when the patients were admitted.36 Other complications, such as infection, malpositioning of the device, subsidence, and perivertebral heterotopic ossification, have low incidence with spinal arthroplasty, especially in the hands of experienced surgeons.37,38 Two long-term studies were recently published describing the clinical and radiographic outcomes of patients more than 10 years postsurgery. The first account by Lemaire et al included 100 patients implanted with the Charité artificial disc, with a mean follow-up of 11.3 years. Authors reported overall 90% good or excellent results with a 92% rate of return to work. At the latest follow-up time point, the range of motion (ROM) at the index level was 10.3 degrees in flexion-extension and 5.4 degrees in lateral bending. Two percent patients presented with adjacent-level disease.39 The second long-term study was published by David. Although the latest results by David further confirmed Lemaire’s excellent outcomes, he did address the fact that surgeon experience greatly impacts clinical outcome, a finding also presented by Regan et al.38 The first clinical series by David—from surgeries performed in 1989, 1990, and 1991—had 69% excellent and good results,40 whereas with more experience, as described recently, his success rate increased to 82.1%.41 In addition, in his latest series, 89.6% patients returned to work. The mean ROM was 10.1 degrees in flexion-extension, and 4.4 degrees in lateral bending. Five “complete ossifications” around prosthesis were observed. Eight patients required revision via posterior instrumented fusion. There were five cases of postoperative facet arthrosis, three cases of subsidence, three cases of adjacent-level disease, and two cases of core subluxation. In addition to these two 10-year studies, the 2-year follow-up randomized controlled IDE studies for both, the Charité and ProDisc-L were recently published, providing additional data on the safety and efficacy of the devices. The Charité IDE trial was described in two recent publications, presenting clinical and radiographic findings.8,13 This study evaluated the lumbar total disc replacement with the Charité artificial disc versus ALIF with BAK and iliac crest autograft. Investigators reported that Charité was a safe and effective alternative to fusion for the surgical treatment of symptomatic disc degeneration in properly selected patients. The Charité group demonstrated statistically significant superiority in two major economic areas, a 1-day shorter hospitalization time, and a lower rate of reoperations (5.4% compared with 9.1%). At 24 months, the investigational group had a significantly higher rate of patient satisfaction (73.7%) than the control group (53.1%, P = 0.0011). This prospective randomized multicenter study also demonstrated an increase in postoperative return-to-work of 9.1% in the investigational group and 7.2% in the control group. Preoperative ROM in flexion/extension was restored and maintained in the Charité patients. In addition, arthroplasty resulted in significantly better restoration of disc space height and lower rate of subsidence than anterior interbody fusion with BAK cages. Clinical outcomes and flexion/extension ROM correlated with surgical technical accuracy of disc placement. A complete radiographic review confirmed that, despite participation of 15 sites and multiple coinvestigators, 83% cases presented with ideal disc placement. At the request of the FDA, clinical and radiographic data from the Charité IDE study patients were further collected to 5 years postoperatively. The 5-year follow-up data were recently compiled and are pending publication.
Lumbar Spine Anatomy and Biomechanics
Indications and Contraindications
Surgical Techniques
Complications
Long-Term Clinical and Radiographic Outcomes

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








