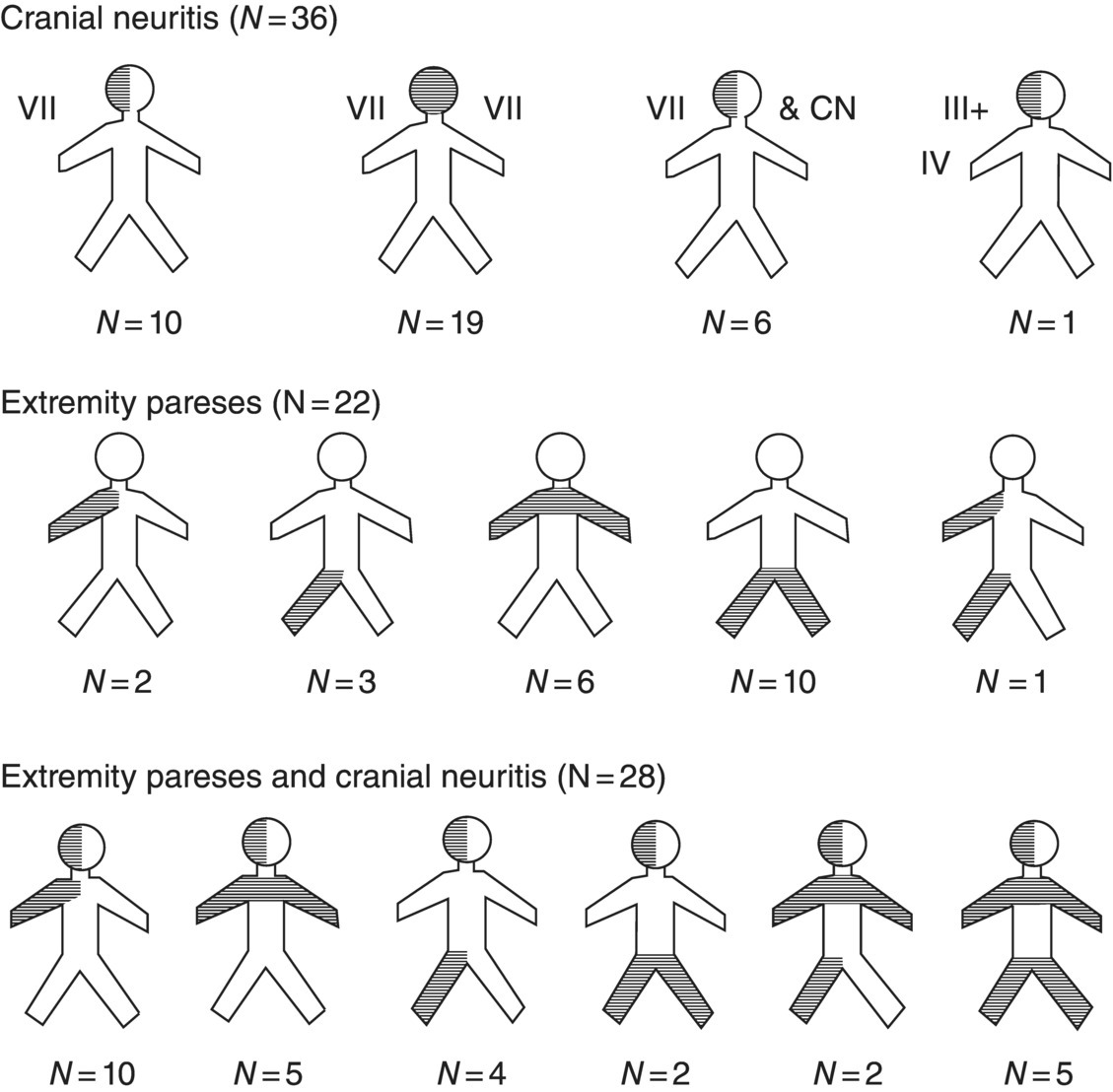
17 Erica Patrick and Eric Logigian Department of Neurology, University of Rochester Medical Center, Rochester, NY, USA Lyme disease, a tick-borne infection caused by the spirochete Borrelia burgdorferi, may cause peripheral or central nervous system (CNS) involvement within days to weeks of onset in 10–15% of patients or months to years of onset in a much smaller percentage. CNS Lyme disease can occasionally mimic multiple sclerosis (MS), and it is important to distinguish the two diseases. This chapter will review nervous system Lyme disease with an emphasis on its diagnosis and treatment. B. burgdorferi sensu stricto is the causative spirochete in North America, while most European cases are caused by Borrelia garinii or afzelii (B. burgdorferi sensu lato). The spirochetal infection is transmitted by the Ixodes tick: Ixodes scapularis in the northeastern and central USA, Ixodes pacificus in the northwestern USA, Ixodes ricinus in Europe, and Ixodes persulcatus in Asia. The basic principles of infection are the same for the different tick species. In order for transmission to occur, the tick must feed on an infected reservoir. For example, in northeastern North America, the white-footed field mouse serves as the reservoir host for the larval and nymphal forms, whereas the definitive host for the adult tick is the white-tailed deer. The spirochetes, which remain in the tick’s gut, will then replicate with continued feeding and ultimately migrate to the tick’s salivary glands for injection into the new host. Transmission of the spirochete occurs slowly. For example, the process requires approximately 48 h of persistent attachment in the case of the I. scapularis tick. Given their smaller size, nymphal ticks are more likely than their adult counterparts to attach for this time period without detection and removal by a human host. Areas endemic for Lyme disease are those in which both the infected reservoir and the Ixodes tick are common and where humans come into contact with the tick. Within the USA, this occurs in rural and suburban areas in the Northeast from Maine to Maryland (particularly coastal regions and the Hudson River valley), in the Midwest in Wisconsin and Minnesota, and in the West in Northern California, and Oregon Lyme disease may also be acquired in central European countries, Scandinavia, Russia, China, or Japan. Spirochetes injected into the skin of a human host migrate centrifugally, resulting in a slowly expanding erythematous lesion known as erythema migrans. This lesion is typically painless and nonpruritic and may expand to a large size. Untreated, erythema migrans lasts on average about 3–4 weeks before subsiding. Flu-like symptoms may accompany or follow erythema migrans due to the early hematogenous dissemination of the spirochete. Patients in this phase may experience fevers, muscle aches, headache, malaise, or fatigue. Once introduced into the human host, the Borrelia spirochete preferentially migrates to certain organs. In addition to the nervous system, the spirochete tends to infect cardiac and rheumatologic tissues. For example, some patients may develop cardiac complications such as atrioventricular block or a subtle myocarditis during the early phase of infection. Rarely, patients with cardiac involvement may develop a dilated cardiomyopathy. In addition to the heart, Borrelia may invade synovial tissue, causing joint inflammation and oligoarticular arthritis, most commonly affecting large joints such as the knee. Occasionally, this results in chronic arthritis. As a general rule, untreated Lyme disease tends to unfold in stages beginning with early localized disease (erythema migrans) followed by early disseminated disease (early neurologic disease, meningitis, cranial neuritis, and radiculoneuritis, and cardiac manifestations, atrioventricular block and myocarditis) and late disease (oligoarticular arthritis and late neurologic manifestations). However, it is important to note that overlap of these stages is not uncommon and that one or more of them may not occur or may not be noticed. Within days to weeks following early dissemination of the Borrelia spirochete, approximately 10–15% of patients develop nervous system involvement. This typically occurs from early spring to late fall with a peak incidence in summer. Early nervous system Lyme disease most commonly presents as lymphocytic meningitis, cranial neuritis, or radiculoneuritis, either alone or in combination (see Figure 17.1). Figure 17.1 The distribution of pareses. Source: Ackerman et al. (1984). Reproduced with permission of Yale Journal of Biology and Medicine. Lymphocytic meningitis often occurs in association with cranial neuritis or radiculoneuritis but can also occur in isolation in approximately 5% of patients, typically 2–10 weeks after onset of infection. As with other forms of meningitis, these individuals may present with a headache, photophobia, or stiff neck. However, meningeal symptoms and signs may be minimal or absent in some patients with Lyme meningitis, and a high index of suspicion is required to perform a lumbar puncture in a patient with other symptoms to suggest the disease. Cerebrospinal fluid (CSF) analysis typically reveals a lymphocytic pleocytosis, an elevated protein, and a normal glucose. Cranial neuropathies are also relatively common during the early phase of Lyme infection. Cranial nerve VII, the facial nerve, is the most frequently affected cranial nerve (seen in about 50–75% of patients with early neurologic involvement) and may be bilateral in about one-third of patients with facial palsy. Other, less commonly involved cranial nerves are the fifth (trigeminal nerve) and sixth (abducens nerve), with rare involvement of the others. The optic nerve, cranial nerve II, is not commonly affected by Lyme disease. However, there are published case reports in Europe and North America describing patients with a painful optic neuropathy that develops along with, or shortly following, symptoms typical of early disseminated Lyme disease. These case reports also suggest that optic neuritis observed in this setting may improve with standard treatment for Lyme disease. Optic neuritis may therefore be a rare complication of early neuroborreliosis, and Lyme disease may be considered in the differential diagnosis in the appropriate clinical setting (e.g., after exposure in an endemic area with other neurologic or nonneurologic symptoms of the disease). An underdiagnosed early manifestation of Lyme disease is radiculoneuritis, which presents as severe radicular pain affecting one or more cervical, thoracic, or lumbosacral dermatomes, often with accompanying motor and reflex changes. This may be confused for a radiculopathy due to structural causes, from diabetes or herpes zoster, or for a primary cardiac or gastrointestinal process. Radiculoneuritis usually presents within 1 month of erythema migrans, if the skin rash is observed, and, like facial palsy, typically resolves spontaneously within several months of onset. Other peripheral nerve manifestations include a confluent or nonconfluent mononeuritis multiplex, brachial neuritis, or, rarely, a progressive demyelinating polyneuropathy typical of Guillain–Barré syndrome (GBS) but with a CSF lymphocytic pleocytosis rather than the typical GBS findings of elevated CSF protein with normal cellularity. Months to years after disease onset, untreated Lyme disease can result in late neurologic manifestations, such as Lyme encephalitis or encephalopathy, encephalomyelitis, or axonal polyradiculoneuropathy. A subacute encephalopathy or encephalitis may develop in patients with a preceding symptomatic Lyme infection. This has been a topic of some controversy, but it seems clear that months to years after onset of infection, a small percentage of untreated patients may develop subacute memory loss, sleep disturbance, irritability, headache, or word-finding difficulty. This syndrome complex is nonspecific, as it may be seen in numerous other systemic diseases or in other diseases of the CNS, such as MS. Patients with Lyme encephalopathy, however, invariably have past or present evidence of Borrelia infection (e.g., erythema migrans, cranial neuropathy or radiculoneuropathy, oligoarticular arthritis), in addition to objective evidence of memory impairment and elevated serum or CSF Lyme titers. It is unclear if Lyme encephalopathy is due to direct Borrelia infection of the brain parenchyma or if the spirochete exerts an extrathecal indirect effect via the diffusion of neuroimmunomodulators into the CSF. Whatever the pathogenesis, these patients respond subjectively and objectively to a 2–4-week course of intravenous (IV) ceftriaxone. In Europe, and less commonly North America, a syndrome of progressive Borrelial encephalomyelitis has been described. This is characterized by direct involvement of the spinal cord or brain parenchyma. This may occur in patients with Lyme radiculitis with spinal cord involvement at the corresponding spinal level, causing symptoms of a myelopathy at and below those levels (i.e., spasticity, a sensory level, or bladder dysfunction). Rarely, Lyme disease may directly affect the brain parenchyma. This typically occurs in focal areas of white matter and may raise the possibility of MS or brain tumor. CSF findings may be similar to those found in MS, except that these patients should have evidence of intrathecal production of anti-Borrelia antibodies. Also, these patients may have had prior symptoms of localized or disseminated Lyme disease. Transverse myelitis is a rare complication of Lyme disease which typically occurs during the later stages, although it has also been reported during the early stage. Affected patients may experience sensory changes, such as band-like tightness, weakness at or below the affected spinal levels, and possibly urinary tract dysfunction. MRI of the spinal cord typically reveals cord edema with or without contrast enhancement of the involved levels. Several case reports note that the MRI scan appears more severe than the clinical presentation. Transverse myelitis from Lyme disease generally responds well to a course of antibiotics. Late peripheral nervous system involvement may also occur. In contrast to the early syndrome of cranial neuritis or radiculoneuritis, later-onset neuropathy is milder and less distinctive with features of a subtle radiculoneuropathy or mononeuropathy multiplex that may become confluent over time. It typically presents with symmetric or asymmetric positive and negative sensory symptoms and signs of distal paresthesias, radicular pain, or both. Weakness, if present, is typically slight. Electrodiagnostic testing typically reveals this to be an axonal polyradiculoneuropathy. This is not considered to be residual disease from an early-onset Lyme radiculoneuritis. There are patients with adequately treated Lyme disease who develop various postinfectious sequelae of Borrelia infection including generalized fatigue, fibromyalgia, headache, cognitive symptoms, or sensorineural hearing loss. This symptom complex is sometimes termed post-Lyme syndrome. Some patients develop diffuse pain and paresthesias with trigger points characteristic of fibromyalgia. In contrast to patients with Lyme encephalopathy, encephalitis, or encephalomyelitis, these patients typically lack objective evidence of nervous system involvement on testing and do not experience sustained improvement after further courses of antibiotic therapy. The diagnosis of neurologic Lyme disease is based on clinical criteria, with confirmatory lab testing. Although there are a multitude of tests that can be used to aide in the diagnosis of Lyme disease, most lack the high sensitivity and specificity expected for an accurate laboratory tool. In the appropriate clinical setting (e.g., a high pretest probability of disease), a positive test is confirmatory. By contrast, if the clinical data yield a low pretest probability, then a positive test result is more likely to be a false positive.
Lyme Neuroborreliosis
Introduction
Borrelia burgdorferi infection
Nonneurologic manifestations
Early neurologic manifestations
Later-onset neurologic manifestations
Diagnosis
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



