6 Malignant Ischemic Stroke Katja E. Wartenberg Malignant strokes are large strokes with edema and swelling that cause secondary injury due to mass effect. Neurologic deterioration occurs as a consequence of cytotoxic edema that causes an early rise in intracranial pressure and subsequent herniation and death. Large anterior circulation (proximal middle cerebral artery [MCA] or internal carotid artery [ICA] territory strokes) or large cerebellar infarcts are an important cause of morbidity and mortality in the neurologic intensive care unit. Malignant infarcts constitute -10% of all ischemic strokes.1 The typical etiology of these infarcts is either cardioembolic or due to large vessel occlusion. Malignant edema is more common in women and is classically described as developing within 24 hours, although edema often develops after 3 to 4 days. Early swelling can be attributed to reperfusion edema or possibly the effects of tissue plasminogen activator (tPA). Though not all patients with massive stroke deteriorate, sensitive predictors of decompensation include high NIH Stroke Scale scores (NIHSS) (Table 6.1), depressed level of consciousness, history of hypertension (HTN) or heart failure, asymmetric pupil size, early nausea and vomiting, younger age (mean age 56 years), coma on the day of admission, poor collateral circulation, and carotid dissection.2 Radiographic predictors include computed tomography (CT) hypodensity of >50% of the affected MCA territory, large magnetic resonance imaging (MRI) diffusion weighted and apparent diffusion coefficient volume, and small perfusion to diffusion mismatch.
| 1a | Level of consciousness 0 = alert, 1 = drowsy, 2 = stuporous, 3 = comatose |
| 1b | Level of consciousness questions 0 = answers both correctly, 1 = answers one correctly, 2 = both incorrect |
| 1c | Level of consciousness commands 0 = obeys both correctly, 1 = obeys one correctly, 2 = both incorrect |
| 2 | Best gaze 0 = normal, 1 = partial gaze palsy, 2 = forced deviation |
| 3 | Visual fields 0 = no visual loss, 1 = partial hemianopsia, 2 = complete hemianopsia, 3 = bilateral hemianopsia |
| 4 | Facial paresis 0 = normal movement, 1 = minor paresis, 2 = partial paresis, 3 = complete palsy |
| 5–8 | Right/left arm/leg motor 0 = no drift, 1 = drift, 2 = some effort v gravity, 3 = no effort v gravity, 4 = no movement |
| 9 | Limb ataxia 0 = absent, 1 = present in 1 limb, 2 = present in 2 or more limbs |
| 10 | Sensory 0 = normal, 1= partial loss, 2 = dense loss |
| 11 | Best language 0 = no aphasia, 1 = mild-moderate aphasia, 2 = severe aphasia, 3 = mute |
| 12 | Dysarthria 0 = normal articulation, 1 = mild-moderate dysarthria, 2 = unintelligible or worse |
| 13 | Neglect/inattention 0 = no neglect, 1 = partial neglect, 2 = complete neglect |
*Total score 0–42; 0 = best, 42 = worst, v = versus
Data from: www.NIHSS.com.
History and Examination
History
- Obtain a timeline for onset of the stroke: establish time to thrombolysis, initial NIHSS, and time of decompensation.
- Check for history of rhythmic movement or subtle twitching that might point to seizure activity.
Physical Examination
Look for signs of neurologic deterioration from cerebral edema and impending herniation after large hemispheric stroke or brainstem compression and/or hydrocephalus after large cerebellar infarction, including:
- Vital signs. Cushing’s triad (hypertension, bradycardia, respiratory irregularity)—classic signs of elevated intracranial pressure (ICP)
- Assess for nausea, vomiting.
- Assess for hyperventilation or hypoventilation, Cheyne-Stokes breathing, and apneustic, cluster, or ataxic respirations.
- Assess for arrhythmia, bradycardia, sustained hypertension, or blood pressure lability.
Neurologic Examination
- With large, anterior circulation malignant strokes, the neurologic examination typically reveals depressed mental status, forced gaze deviation, aphasia or neglect, visual field defects, and/or hemiparesis.
- In posterior circulation strokes, look for crossed cranial nerve and long track signs, quadriparesis, depressed mental status, multiple cranial neuropathies, abnormal pupils, ataxia, dysarthria, abnormal brainstem reflexes (corneal, oculocephalics, gag), nausea/ vomiting (due to either elevated ICP or irritation of the area postrema at the floor of the fourth ventricle), downward gaze deviation (sign of hydrocephalus due to enlarged third ventricle and compression of the rostral intermedial longitudinal fasciculus), and/or elevated tone in the lower extremities (early indication of hydrocephalus).
- Assess for subtle twitching or eye deviation away from the side of the lesion that might indicate seizure activity.
- Assess for impending herniation:
- First sign is usually drowsiness, followed by progressive obtundation to stupor or coma.
- Pupillary asymmetry, ocular deviation (unilateral oculomotor palsy from uncal herniation or traction on the third nerve)
- Contralateral motor posturing (decorticate = flexor posturing, decerebrate = extensor posturing) or Kernohan’s notch phenomenon (long track signs/weakness ipsilateral to the lesion due to herniation and compression of the contralateral cerebral peduncle)
- Bilateral motor posturing and lower extremity rigidity
- First sign is usually drowsiness, followed by progressive obtundation to stupor or coma.
- The NIH Stroke Scale is commonly used to follow the neurological exam (Table 6.1).
Differential Diagnosis
- Development of cerebral edema with tissue shifts, eventually resulting in herniation
- Intracranial bleeding (e.g., after thrombolytic therapy)
- Hydrocephalus after cerebellar infarction
- Ongoing ischemia. Exclude hypoperfusion in large artery stenosis/occlusion, ongoing embolization from a cardiac or vessel source (i.e., dissection—look for headache; neck, eye, or face pain; recent stress on neck; cranial neuropathies), progression of posterior circulation thrombosis (lethargy/coma, top of the basilar syndrome, cranial neuropathies with crossed long track signs, cortical blindness, cerebellar findings), or vascular compression of the anterior and posterior cerebral arteries against the falx or tentorium (rare; due to swelling and mass effect).
- Seizure. Perform continuous electroencephalogram (EEG) monitoring; consider antiepileptic drugs
- Sedating drugs—discontinue
- Fever (see Chapter 20)
- Hypo- or hyperglycemia. Correct and maintain strict normoglycemia.
- Hypoxia, hypercarbia. Check arterial blood gas (ABG), intubate if necessary, adjust ventilator settings
- Metabolic causes. Renal or hepatic insufficiency → check laboratories, including electrolytes, determine underlying cause, and treat accordingly.
- Infection. Pneumonia, urinary tract infection, meningitis/ventriculitis (after neurosurgical procedures), line infection, sinusitis, intra-abdominal abscess → check complete blood count (CBC), C-reactive protein (CRP), procalcitonin, chest radiograph (CXR), urine analysis, blood-, urine-, and deep tracheal aspiration cultures. Treat ventilator-associated pneumonia and sepsis with a combination of broad-spectrum antibiotics. Change central lines if appropriate.
Life-Threatening Diagnoses Not to Miss
- Herniation
- Obstructive hydrocephalus from edema
- Intracranial hemorrhage/hemorrhagic conversion
- Ongoing ischemia
- Status epilepticus
Diagnostic Evaluation
- Laboratory studies
- Evaluate CBC, electrolytes, liver function, glucose level, coagulation studies, urine toxicology screening if appropriate, antiepileptic drug (AED) levels if on AED and suspicion of seizure, ABG.
- In febrile patients, check urine analysis; blood-, urine-, and deep tracheal aspiration cultures; CXR; CRP; procalcitonin; further detailed search for infection focus if appropriate.
- Evaluate CBC, electrolytes, liver function, glucose level, coagulation studies, urine toxicology screening if appropriate, antiepileptic drug (AED) levels if on AED and suspicion of seizure, ABG.
- Imaging studies
- Immediate noncontrast CT scan: mass effect on CT such as compression of the frontal horn, shift of the septum pellucidum, and later shift of pineal gland are worrisome features for impending clinical deterioration or herniation.3
- CT perfusion, CT angiogram, and/or MRI, MR angiogram, MR perfusion are useful if the CT scan does not reveal the cause of neurologic deterioration and/or ongoing ischemia is suspected.
- Digital subtraction angiography can be useful to assess the etiology of stroke and collateral circulation, particularly if ongoing ischemia or hypoperfusion is suspected.
- Immediate noncontrast CT scan: mass effect on CT such as compression of the frontal horn, shift of the septum pellucidum, and later shift of pineal gland are worrisome features for impending clinical deterioration or herniation.3
- Other diagnostic studies
- Consider continuous EEG monitoring.
- Transcranial Doppler ultrasound to determine if the ICA, MCA, or basilar artery is open.
- ICP, brain oxygen monitoring, or microdialysis may be considered to monitor treatment of cerebral edema in large hemispheric strokes.
- Consider continuous EEG monitoring.
Treatment
Initial Management
Revascularization should be considered at presentation for patients with large ischemic strokes. This constitutes intravenous (IV) rtPA/alteplase (0.9 mg/kg IV, 10% as an initial bolus over 1 minute, and the rest as an infusion over the remaining hour, maximum dose: 90 mg) in patients who present within a 3-hour time window of ischemia onset (American Heart Association/American Stroke Association [AHA/ASA] class I, level A)4,5 and do not have any contraindications (Table 6.2).4
| Onset of symptoms >3 h before beginning treatment |
| Head trauma or prior stroke in previous 3 months |
| Myocardial infarction in previous 3 months |
| GI or urinary tract hemorrhage in previous 21 d |
| Major surgery in previous 14 d |
| Arterial puncture at noncompressible site in previous 7 d |
| History of previous ICH |
| Blood pressure >185/110 mm Hg after attempted BP treatment |
| Evidence of active bleeding or acute trauma (fracture) |
| Anticoagulant use or INR >1.7 |
| Received heparin in last 48 h and PTT elevated |
| Platelet count <100,000 |
| Blood glucose <50 mg/dL (new guidelines have no upper glucose limit) |
| Seizure with postictal residual neurologic impairments (relative contraindication) |
| Neurologic signs clearing spontaneously (relative contraindication) |
| Neurologic signs minor or isolated (relative contraindication) |
| Symptoms suggestive of SAH |
| CT with hemorrhage |
| Caution in patients with major deficits |
| Caution if CT shows multilobar infarction with major hypodensity > one-third cerebral hemisphere (check time of onset) |
Abbreviations: AHA, American Heart Association; ASA, American Stroke Association; BP, blood pressure; CT, computed tomography; GI, gastrointestinal; ICH, intracranial hemorrhage; INR, international normalized ratio; IV, intravenous; PTT, partial thromboplastin time; SAH, subarachnoid hemorrhage; tPA, tissue plasminogen activator.
Data from: Adams HP Jr, del Zoppo G, Alberts MJ, et al. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke 2007;38(5):1655–1711.
Manno EM, Nichols DA, Fulgham JR, Wijdicks EF. Computed tomographic determinants of neurologic deterioration in patients with large middle cerebral artery infarctions. Mayo Clin Proc 2003;78(2):156–160.
- There is no upper size limit, age limit, or NIHSS limit for the administration of IV thrombolysis. Patients with seizure at the time of onset of stroke may be eligible for IV tPA treatment if the physician is convinced that the residual impairments are due to stroke and not a postictal phenomenon (AHA/ASA class IIa, level C).5 Blood pressure should be controlled prior to and after tPA administration according to Table 6.3.5
- In patients who do not receive thrombolysis, the treatment threshold for lowering blood pressure (BP) acutely after ischemic stroke is 220/120 mm Hg (AHA/ASA class I, level C).5
- In patients who receive IV tPA, if the patient develops severe headache, acute hypertension, nausea, or vomiting, IV tPA should be discontinued and an emergency CT scan obtained and emergent reversal should be given (see Chapter 4). Physicians should be aware of the complication of angioedema that can cause partial airway obstruction. After tPA administration, BP should be measured every 15 minutes for the first 2 hours and then every 30 minutes for the next 6 hours. Placement of nasogastric tubes, indwelling bladder catheters, or intra-arterial catheters should be deferred. A follow-up CT scan should be obtained at 24 hours before starting anticoagulants or antiplatelet agents.5
- Patients who are not within the time window or are not eligible for IV tPA (i.e., recent surgery) may be eligible for intra-arterial thrombolysis (within 6 hours for the anterior circulation [AHA/ASA class I, level B]5,6 and up to 24 hours in the posterior circulation) or mechanical thrombectomy within 8 hours (class IIb, level B)5,7,8 in selected centers for patients without contraindications. The availability of intra-arterial treatment should not preclude the use of IV tPA in eligible patients (class III, level C).5
- In the ECASS III trial, a randomized, placebo controlled trial of IV tPA versus placebo administered in a 3–4.5 hour time window, patients receiving IV tPA had significantly improved clinical outcome at 3 months. Currently, IV tPA is not FDA approved for a 3–4.5 hour time window.9
| Timing | Blood Pressure | Treatment |
| Eligible for tPA or other acute reperfusion intervention | SBP>185 mm Hg or DBP>110 mm Hg | Labetalol 10–20 mg IV over 1–2 min, may repeat X1 or Nitropaste 1–2 inches or Nicardipine infusion, 5 mg/h, titrate up by 0.25 mg/h at 5–15 min intervals (maximum 15 mg/h); when desired BP attained, reduce to 3 mg/h If BP does not decline and remains >185/110 mm Hg, do not administer rtPA |
| After tPA given | SBP 180–230 mm Hg or DBP 105–120 mm Hg | Labetalol 10 mg IV over 1–2 min, may repeat every 10–20 min (maximum 300 mg) or Labetalol 10 mg IV followed by an infusion at 2–8 mg/min |
| SBP>230 mm Hg or DBP 121–140 mm Hg | Labetalol 10 mg IV over 1–2 min, may repeat every 10–20 min (maximum 300 mg) or Labetalol 10 mg IV followed by an infusion at 2–8 mg/min. or Nicardipine infusion, 5 mg/h, titrate up by 2.5 mg/h at 5–15 min intervals (maximum 15 mg/h) | |
| If BP not controlled | Consider sodium nitroprusside* |
Abbreviations: BP, blood pressure; DBP, diastolic blood pressure; IV, intravenous; SBP, systolic blood pressure; tPA, tissue plasminogen activator.
Data from: Adams HP Jr, del Zoppo G, Alberts MJ, et al. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke 2007;38(5):1655–1711.
*Nitroprusside can raise ICP and cause cyanide toxicity
Medical Management
- Mechanical ventilation is recommended for patients with acute stroke who have a decreased level of consciousness or bulbar dysfunction causing airway compromise (AHA/ASA class I, level C).5
- Cerebral edema and elevated ICP or shift should be treated (see Chapter 15). It should be noted that many patients with malignant stroke herniate with normal ICP values.10 This occurs due to pressure gradients. Aggressive management of edema should occur in deteriorating patients, even if the ICP is “normal.” The AHA/ASA recommends measures to lessen the risk of edema and close monitoring for neurologic deterioration during the first few days after stroke (class I, level B). There is limited evidence to show that aggressive medical management of edema improves outcomes (class IIa, level C),5 but such treatment is reasonable, particularly as a bridge to decompressive hemicraniectomy. Hyperventilation should be used only for a short period of time or during frank herniation. Steroids have not been shown to be useful for edema related to ischemic stroke and are not recommended (class III, level A).5
- Anticonvulsants. Prophylactic anticonvulsants are not recommended in large hemispheric or cerebellar stroke (class III, level C).5 However, seizures (incidence 2 to 33% after large hemispheric stroke) may worsen cerebral edema, increase ICP, and induce neurologic deterioration. Treatment with phenytoin, fosphenytoin, or levetiracetam can be considered in these cases. Continuous electroencephalographic monitoring (cEEG) may be helpful in patients in persistent stupor or coma to enable detection and treatment of nonconvulsive seizures or status epilepticus. Patients who have experienced a seizure should be treated with anticonvulsants.
- Fever. Persistent fever after stroke impacts mortality and outcome. Even small temperature elevations have been shown to worsen neuronal injury and increase mortality. Therefore, fever should be treated aggressively (see Chapter 20). According to the AHA/ASA guidelines,5 sources of fever should be treated and antipyretic medications should be administered to lower temperature in febrile patients with stroke (class I, level C). Prophylactic antibiotics are not recommended (class III, level B).5 At this time, insufficient evidence exists to recommend induced hypothermia for the treatment of patients with acute stroke (class III, level B).5 Induced hypothermia may be considered as part of an ICP management algorithm (see Chapter 15).
- Cardiac monitoring should be performed during at least the first 24 hours after ischemic stroke (AHA/ASA class I, level B).5
- Nutrition and glucose control. Patients who cannot consume food orally should receive nasogastric, nasoduodenal, or percutaneous endoscopic gastrostomy tube (PEG) feedings. The timing of PEG placement is uncertain (class IIa, level B).5 Small-bore nasoduodenal feeding tubes are preferred to lower the risk of aspiration. Nutritional supplements are not needed (class III, level B).5 As hyperglycemia may worsen outcome after brain injury, glucose- or dextrose-containing solutions should be avoided. Persistent hyperglycemia should be treated vigorously by means of an insulin protocol utilizing continuous insulin infusions. The ideal glucose target is currently an area of investigation (see Chapter 19). The AHA recommends that glucose control be initiated for serum glucose levels of “possibly 140–185 mg/dL” (class IIa, level C).5
- Deep venous thrombosis prophylaxis. Subcutaneous anticoagulants are recommended in immobilized patients (class I, level A). The use of intermittent compression devices is recommended in patients who cannot receive subcutaneous anticoagulants (class IIa, level B)5 (see Chapter 18).5
Surgical Management
Cerebellar Stroke
Large cerebellar strokes can develop not only surrounding edema leading to brainstem compression, but can also cause obstructive hydrocephalus due to compression of the fourth ventricle. Suboccipital craniectomy, cerebellectomy, or external ventricular drainage (EVD) are indicated for:
In a series of 50 patients with cerebellar stroke,10 the indication for selected surgical procedures was stratified as follows:
- Complete effacement of the fourth ventricle → decompression and EVD
- Fourth ventricle open, not completely effaced, Glasgow Coma Score (GCS) normal → medical management of cerebral edema
- Fourth ventricle open, not completely effaced, GCS deteriorating:
- With hydrocephalus → EVD
- No hydrocephalus → decompression
- With hydrocephalus → EVD
According to ASA/AHA guidelines, patients with acute hydrocephalus after cerebellar stroke can be treated with an EVD, though upward herniation is a concern (class I, level B). Decompressive evacuation of a space-occupying cerebellar infarction is potentially life saving, can treat both hydrocephalus and brainstem compression, and is recommended for patients with major cerebellar infarctions (class I, level B).5
Decompressive Surgery for Large Hemispheric Stroke
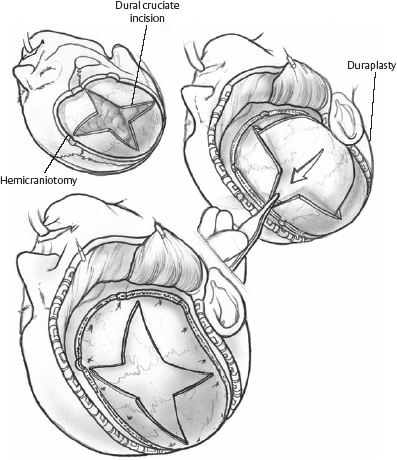
Hemicraniectomy for large hemispheric infarction has been performed for decades. Horizontal and vertical tissue shifts and ventricular and vascular compression by massive brain edema are relieved by removal of the bone flap over the frontal, temporal, and parietal lobe at the infarct site. This allows the edematous brain to expand extracranially, improves cerebral perfusion pressure (CPP) and retrograde flow in the MCA, preserves cerebral blood flow (CBF), and may prevent further ongoing ischemia. The diameter of the craniectomy should be at least 12 cm (14 to 15 cm anterior-posterior, and 10 to 12 cm from the temporal base to the vertex is recommended). A small diameter hemicraniectomy can result in compression and kinking of bridging veins, or mushroom-like herniation of the brain with shearing distortion and additional ischemic injury (Fig. 6.1). Resection of the infarction is not advisable, as the margins between infarct and penumbra are poorly defined, however tissue resection is occasionally required in cases of extreme swelling when skin closure is difficult. The bone flap can be conserved in the abdominal subcutaneous tissue or in a cooled sterile isotonic solution. Synthetic bone flaps can be constructed as an alternative. Reimplantation of the bone flap is possible 6 to 12 weeks after removal, once the swelling has resolved. Potential complications include intracranial, wound, and bone flap infection, subdural and epidural hematoma, subdural cerebral spinal fluid (CSF) hygroma, paradoxical herniation after the swelling period, and persistent hydrocephalus linked to delayed replacement of the bone flap.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








