(1)
Department of Neurosurgery, Keio University School of Medicine, Shinjuku-ku, Tokyo, Japan
Abstract
Macroscopically, hemangiopericytoma (HPC) and meningioma are similar. However, treatment strategies for these tumors sometimes differ because of differences in their clinical behavior and structure, principally their vascularity.
HPC must be totally resected at initial surgery, which sometimes requires preoperative embolization, and close radiographic follow-up is mandatory for detection of local recurrence or metastasis. In the case of residual tumors, adjuvant radiotherapy including conventional radiotherapy, stereotactic radiosurgery, and stereotactic radiotherapy should be deployed. Repeat surgery or salvage radiotherapy is effective. Although chemotherapy has not shown definitive efficacy until date, novel approaches for systemic metastasis that cannot be controlled by present modalities are expected.
Introduction
Intracranial hemangiopericytoma (HPC) is an aggressive tumor with macroscopic and radiographic features that are similar to those of meningioma; however, there are distinct differences in the biology and clinical course of these two tumors. HPC is a rare tumor that accounts for 2.5% of all meningeal tumors and <1% of all intracranial tumors (Schiariti et al. 2011). In contrast, meningioma is one of the most frequent primary brain tumors. There is little difference in the gender incidence of HPC, with only a slight male predominance (Lamar and Lesser 2011). HPC occurs most frequently during the fifth decade of life.
HPC can arise in any part of the human body; however, it is most common in the lower extremities and retroperitoneum. Intracranial or meningeal HPCs usually arise from the meninges of the falx, tentorium, and dural sinuses as meningiomas. Supratentorial lesions are more common than infratentorial or spinal lesions (Sibtain et al. 2007). HPC can also occur in the third or lateral ventricle (Abrahams et al. 1999), the pineal region (Stone et al. 1983), Meckel’s cave (Muto et al. 2010), and the sellar region (Juco et al. 2007).
Historically, HPC was first described as a primarily soft tissue tumor arising from pericytes, which are contractile spindle cells surrounding capillaries and postcapillary venules (Stout and Murray 1942). The term “meningioma” was first used to define whole tumors arising from the meninges, including tumors originating from meningothelial (arachnoid) and other mesenchymal cells, such as the angioblastic meningioma first described by (Bailey et al. 1928).
Begg and Garret (1954) reported occurrence of HPC in the meninges and termed such masses “meningeal HPC.” However, there has long been controversy as to whether these are meningioma variants of the angioblastic meningioma first defined by (Cushing 1938).
After much discussion, the WHO classification was revised in 1993 (Kleihues and Scheithauser 1993) and tumors arising from the meninges but not originating from arachnoid cells were excluded from meningioma and classified into another category. Since this revision, meningeal HPC has been recognized as an independent category with a different origin, biological behavior, and optimal treatment compared with meningioma.
Pathological Findings
Macroscopically, HPC is a firm and nodular tumor attached to the meninges with a clear margin to the cortical surface. It cannot be distinguished from meningioma, except for a high bleeding tendency. Microscopically, a characteristic staghorn pattern of thin-walled vessels, which is the hallmark of HPC tumors, is observed (Middleton et al. 1998). Tumor cells are uniform and polygonal to spindle shaped, often with vesicular nuclei. Reticulin staining reveals a typical pattern of fine fibers surrounding the individual tumor cells (Middleton et al. 1998). HPCs, like meningiomas, are positive for vimentin and CD34. Epithelial membrane antigen (EMA) is generally negative, but focal reactivity for EMA is sometimes found in HPC (Rajaram et al. 2004). Immunohistochemical expression of the vascular endothelial growth factor receptor (VEGFR) has been reported in HPC tumor cells and in the endothelium, with overexpression of vascular growth factors and receptors such as VEGFR1 and VEGFR2 in the endothelium (Hatva et al. 1996). A study by Dietzmann et al. (1997) showed that HPCs frequently overexpress the platelet-derived growth factor receptor (PDGFR), which is detectable by immunohistochemistry.
Radiographical Features
On computed tomography (CT) imaging, hyperostosis or calcification, which are frequently observed in meningiomas, are seldom observed in HPCs (Chiechi et al. 1996; Barba et al. 2001). Instead, bony erosion is often found (Chiechi et al. 1996).
In magnetic resonance (MR) studies, HPCs are typically isointense to grey matter on T1- and T2-weighted images (Sibtain et al. 2007). The intratumoral flow voids suggest high vascularity, which is sometimes found in meningiomas but is more frequent in HPCs (Chiechi et al. 1996; Sibtain et al. 2007).
On angiography, some angioarchitectural patterns have been shown to be common in HPCs and are useful for distinguishing them from meningiomas [19]. HPC is supplied from the internal carotid artery (ICA) or vertebral artery (VA), and external carotid arteries (ECA), with dominant supply from the ICA branches rather than ECA, which is commonly seen in meningiomas. There are many intratumoral, irregular, corkscrew vessels arising from a main feeder; intense fluffy tumor staining rather than the sunburst pattern observed with meningiomas; no early draining veins; and a prolonged tumor circulation time (Marc et al. 1975; Sibtain et al. 2007).
Clinical Course
HPCs have a peculiar biological behavior and prognosis with local aggressiveness, a high rate of recurrence, and a propensity to metastasize to numerous extracranial locations.
Rutkowski et al. (2010) analyzed 277 patients with HPC and reported an overall median survival of 13 years, with 1-, 5-, 10-, and 20-year survival rates of 95, 82, 60, and 23%, respectively. Schiariti et al. (2011) reported overall survival rates of 93, 67, 45, and 23% at 5, 10, 15, and 20 years, respectively. Ecker et al. (2003) reported that the 5-year Kaplan–Meier survival rate among patients treated since 1990 was 93%. The 5-year disease-free survival rate was 89%.
For local control, which is influenced by treatment strategy, the 5-, 10-, and 15-year recurrence rates after surgery without radiation have been reported to be 54, 92, and 100%, respectively (Schiariti et al. 2011).
Many patients develop distant metastases. Because of the small number of cases, the rate of metastasis varies. Though some authors have reported around 20% metastasis (Rutkowski et al. 2012; Olson et al. 2010; Schiariti et al. 2011), Soyuer et al. (2004) reported 5-, 10-, and 15-year distant metastasis-free survival rates of 80, 46, and 21%, respectively. Adjuvant radiation seems to decrease local recurrence, as is discussed later; however, it has no effect on the incidence of metastasis (Dufour et al. 2001; Rutkowski et al. 2012; Schiariti et al. 2011). Distant metastasis was correlated with shorter survival (Kano et al. 2008). Until date, there has been no report of an effective prophylaxis for preventing distant metastasis. The incidence of recurrence or metastasis increases with duration of follow-up (Olson et al. 2010), emphasizing the necessity of long-term follow-up.
Presurgical Embolization
HPC usually has multiple feeders and plenty of intratumoral vascular networks, and thus, presurgical embolization is required. In general, feeder occlusion and intratumoral embolization must be differentiated. Proximal feeder occlusion by detachable coils or concentrated glue is generally safe and easy, especially in ECA feeders. However, proximal feeder occlusion of ECA feeders, which is sometimes useful in meningioma surgery, has little effect on decreasing intraoperative blood loss in cases where cortical (ICA) or vertebral–basilar artery (VA-BA) feeders dominate supply to the tumor. A combination of embolization of intracranial feeders (ICA, VA-BA) and surgical detachment of ECA feeders might be useful, although embolization of intracranial feeders carries greater risks than that for ECA feeders. Ideally, embolization of the tumor bed by particles or liquid materials is required to decrease intraoperative blood loss. However, in cases of hemangioblastomas, which are also rich in vascularity and sometimes contain arteriovenous (AV) shunt-like intratumoral vessels, tumor bleeding after particle embolization has been reported (Cornelius et al. 2007). As in our case 1 (Fig. 4.1), almost complete embolization can make surgical resection safer and more comfortable and successful. However, tumor bleeding and swelling that worsens the preexisting mass effect of the tumor could sometimes lead to a disastrous situation requiring emergent decompression.
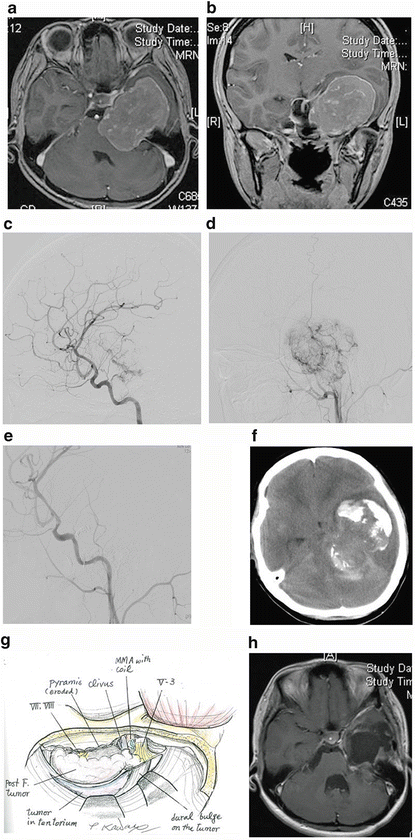

Fig. 4.1
Case 1. A 20-year-old female complained of gait disturbance. Preoperative gadolinium-enhanced MRI of (a) axial and (b) coronal images showed a dumbbell-type tumor severely compressing the brainstem. Preoperative angiography via (c) ICA and (d) ECA injection showed marked staining from many feeder branches with intratumoral corkscrew vessels. (e) Embolization of the tumor and its feeder vessels with polyvinyl alcohol with tantalum powder and detachable coils led to nearly complete disappearance of the tumor stain on common carotid angiography. (f) However, CT revealed a subarachnoid hemorrhage and worsening of peritumoral edema 6 h after embolization. We subsequently performed an emergency tumor removal. (g) A transpetrosal approach with osteotomy of the zygomatic arch was selected to obtain direct access and a wider operative field as well as a view of the superior part of the tumor. Drilling of the petrous apex was not necessary because of bone erosion from the tumor. Bleeding from the tumor was not prominent, and softening of the tumor made resection easier than expected. GTR was achieved. The trochlear and abducens nerve could not be preserved because of total encasement by the tumor. (h) Postoperative MRI showed no residual tumor. Recurrence has not been detected for 41 months
Thus, presurgical embolization can be considered in the case of proximal occlusion of a feeding artery that cannot be accessed in the early stages of surgery or tumor bed embolization can be achieved using liquid formulations such as n-butyl cyanoacrylate (NBCA) or Onyx which rarely induces recanalization after embolization. And care should be taken not to avoid pressure injection of contrast materials or embolic agents. Preparations should also be made for emergency situations requiring surgery for worsening peritumoral edema or tumor bleeding after embolization.
Surgery
Surgery represents the mainstay treatment for HPC, especially in the initial setting. It not only offers immediate alleviation of the mass effect but also allows pathological confirmation, thus facilitating differential diagnosis of HPCs from meningiomas.
Most importantly, the greater the extent of resection, the better the prognosis. In a previous study, local control rates [5-year local control rates for patients treated with gross total removal (GTR) and subtotal removal (STR)] were 84 and 38%, respectively (P = 0.003) (Soyuer et al. 2004). GTR was also associated with increased overall survival (log-rank, P < 0.05) (Rutkowski et al. 2012) compared with STR (Rutkowski et al. 2010). Although some reports have not reported any benefit from radiation (Rutkowski et al. 2012), consistent benefits have been reported for resection with GTR (Schiariti et al. 2011).
Surgical Concept
As mentioned previously, obtaining a definitive preoperative diagnosis from radiologic findings alone is sometimes difficult as discriminating meningioma from HPC is not easy. Thus, in practice, the strategy for initial surgery is based on that for meningioma. However, we have to bear in mind that HPC is richer in vascularity and is fed by ICA or VA-BA in addition to ECA; furthermore, it has a higher rate of local recurrence than meningioma. HPC surgery is therefore sometimes more challenging than meningioma surgery. The surgical approach chosen must ensure both a wider operative field and easy accessibility to the majority of feeders. In meningioma surgery, the shortest and most direct approach to the point of tumor attachment should be the priority.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






