Trauma is a leading cause of death and disability in the United States. Injury to the central nervous system peaks at ages 15 to 24 years but is also higher in children less than 5 years of age and adults over 75 years. Males are more commonly affected than females and racial differences are seen as well. By conservative estimates, ~500,000 traumatic head injuries occur yearly, constituting a significant public health issue. Over 17,000 of the most severe result in death.1 Many more patients survive with significant disability. Acute and chronic care of patients suffering central nervous system trauma constitutes a significant proportion of health care expenditures, not to mention the cost to their families and society. The Brain Trauma Foundation and the Neurotrauma Foundation, in concert with the American Association of Neurological Surgeons and the Congress of Neurological Surgeons, have been instrumental in forming evidence-based consensus groups to comprehensively review the available literature and provide standards, guidelines, and options for the medical and surgical management of severe closed head injury as well as penetrating head injury.2,3 These in-depth reviews exposed a mixed group of well-founded practices along with frequently used but unproven interventions, thus giving rise to strong recommendations regarding current practice and for future areas of research. These efforts led the way for the entry of neurosurgical practice into the evidence-based era of medical care. The Glasgow Coma Scale (GCS) (Table 5–1) has been universally accepted as a reliable, objective measure of level of consciousness and head injury severity almost since its introduction by Teasdale and Jennett three decades ago.4 Although there have been several studies correlating the GCS score to outcome, there have been comparatively few on intra- and interrater reliability of this commonly used clinical tool. A National Library of Medicine (MEDLINE) literature search from 1974 to present yielded six abstracts and articles on the reliability of the GCS. In 1978, Teasdale et al5 reported on the “practical reliability” of the GCS in clinically assessing individual patients across multiple observers. The disagreement rates from rater to rater were small, ranging from 0.089 for eye opening to 0.191 for motor response. This interrater disagreement rate was devised specifically for this study and thus cannot be compared with widely accepted methods of determining reliability such as the kappa statistic.5 Menegazzi et al6 did use kappa statistic to determine the reliability of the GCS using paramedics and emergency physicians as raters. Interrater kappa values (agreement beyond chance) of 0.48 were demonstrated for a GCS score <9, 0.34 for a GCS score of 9 to 12, and 0.85 for a GCS score of 13 to 15. The emergency physicians had intrarater values of 0.66, the paramedics, 0.63. Thus, there was excellent interrater reliability only for a GCS score of 13 to 15, and moderate intrarater reliability across all groups.  5
5 
Management of Neurotrauma
Jack E. Wilberger
Patient Assessment
| Eye Opening | |
| Spontaneous | 4 |
| To verbal command | 3 |
| To pain | 2 |
| None | 1 |
| Best Motor Response | |
| Obeys commands | 6 |
| Localization of painful stimulus | 5 |
| Flexion-withdrawal response to pain | 4 |
| Abnormal flexion response to pain (decorticate posturing) | 3 |
| Extension response to pain (decerebrate posturing) | 2 |
| None | 1 |
| Best Verbal Response | |
| Oriented conversation | 5 |
| Disoriented conversation | 4 |
| Inappropriate words | 3 |
| Incomprehensible sounds | 2 |
| None | 1 |
Establishing the Diagnosis
The introduction of computed tomography (CT) in 1972 allowed for the first time the direct visualization of the brain and specific delineation of traumatic abnormalities (Figs. 5–1, 5–2). Given its revolutionary nature, it was impossible to perform comparative studies with previously available diagnostic tests such as pneumoencephalography and angiography. There has been, however, extensive investigation of the prognostic significance of various traumatic CT abnormalities.7
Despite the introduction of magnetic resonance imaging (MRI), CT has remained the primary diagnostic test in the acute evaluation of head injury (Fig. 5–3). Multiple studies have demonstrated the superiority of MRI over CT in the detection of traumatic abnormalities such as diffuse axonal injury.8 However, there has never been a study of MRI in the acute setting after severe head injury (HI). Indeed, in the various comparative studies undertaken, CT has been found to be superior in delineating the types of acute hemorrhagic lesions important in early management of severe HI (Fig. 5–4).9,10
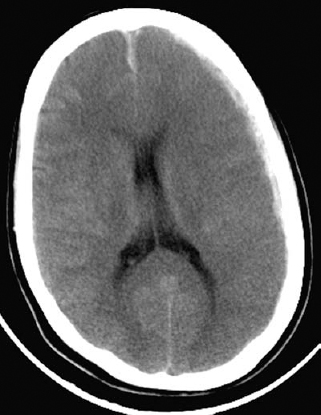
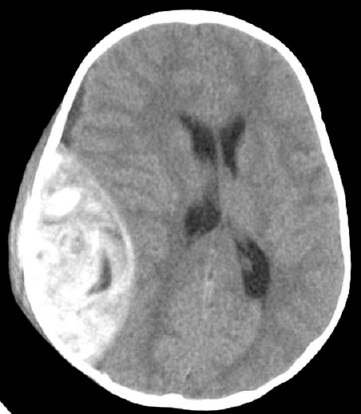
Figure 5–1 A computed tomographic scan showing a small acute subdural hematoma.
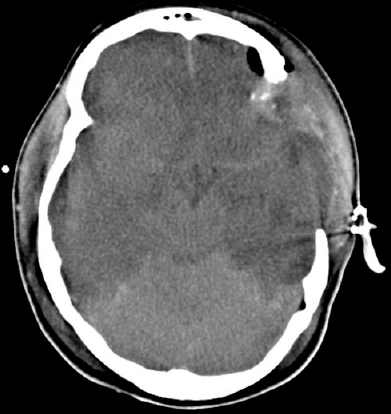
Figure 5–2 A computed tomographic scan showing an acute epidural hematoma.
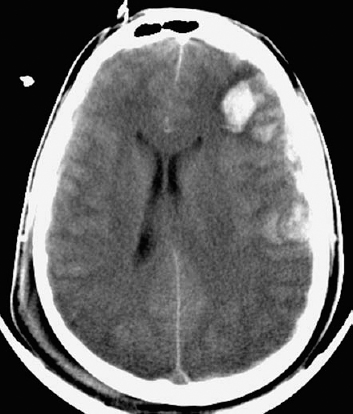
Figure 5–3 A computed tomographic scan showing cerebral contusions.
Figure 5–4 This computed tomographic scan is of the patient shown in Figure 5–1 taken 3 days later. It shows marked cerebral edema that proved fatal despite decompressive craniectomy.
Determination of Prognosis
In neurotrauma, arguably more so than in any other area of neurosurgical endeavor, extensive efforts have been expended to identify those factors singly or in combination that would reliably predict outcome after severe HI. They include the GCS, age, pupillary response, physiological variables, intracranial pressure, CT, MRI, evoked potentials, cerebrospinal fluid and genetic markers.
With the publication of Bullock et al’s7 Guidelines for the Management of Severe Brain Injury, an evidence-based approach was developed to evaluate the available literature on early clinical factors—the GCS, age, pupillary diameter and light reflex, hypotension and hypoxia, CT findings—and to determine sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) with respect to utilization in outcome prediction.
Utilizing this methodology in the evaluation of over 200 articles identified in an extensive MEDLINE literature search, only bilaterally absent pupillary reflexes, hypotension combined with hypoxia, CT-identified midline shift >5 mm, or the presence of a mass lesion in patients over 45 years old had a PPV of >70%. In other words, if one of these factors is present then there is a >70% probability of a poor outcome. This is in contrast to the finding of a continuous, stepwise increasing probability of poor outcome with decreasing GCS and advancing age.
Other investigators have attempted to define various combinations of clinical factors in an attempt to improve sensitivity and PPV for outcome prognostication. The Leeds prognostic score includes a variety of clinical factors, intracranial pressure (ICP), and CT findings to create a 24-point scale. Among 239 patients studied, there were no survivors with a score ≥13.11 The validity of the Leeds score was challenged in a subsequent study by Feldman et al,12 finding an incorrect prediction of fatality in almost one third of the 610 patients studied. Quigley et al13 found the combination of the GCS score and age to predict outcome accurately in 380 very severe head-injured patients (a GCS score of 3 to 5). The oldest functional survivor with a GCS score of 3 was <40; a GCS score of 4 <50; and a GCS score of 5 <60.13 These findings, however, have yet to be replicated.
Evaluation of Intervention
Prevention
Although typically “prevention” is considered in the context of ameliorating secondary injury to the brain, a considerable effort has been undertaken in the development of primary head injury prevention programs. The Think First program has been embraced by Neurosurgery since 1986. The fundamental goal of Think First is to educate young people on the causes and consequences of a head injury. The premise of the program is that increased knowledge and awareness will lead to a decrease in the types of behaviors that may predispose to these injuries. Programs are now available for elementary as well as middle school with over 750,000 children and teens participating in 2003.
The evaluation of the efficacy of Think First, as well as several other prevention programs, has been limited by available methodology. Pre-and postprogram surveys regarding safety knowledge and self-reported risk-taking behaviors have consistently shown significant improvements. However, these have generally been performed immediately and at most several months after the program.14,15 One study found no significant short-term change in behavior—wearing bicycle helmets or seat belts—after a single exposure to the Think First program.15 Thus, the true contribution of Think First in decreasing the incidence of head injury in its target population remains an unanswered question.
Treatment Efficacy
In contrast, there has been extensive investigation, including a large number of randomized clinical trials, on therapies to prevent secondary injury. Although no study to date has shown a significant improvement in overall outcome, subgroup analyses have provided some suggestion that certain patients/injury types may benefit from specific therapeutic interventions. Hypothermia provides an excellent example in this regard.
Hypothermia may attenuate brain injury, especially during periods of absolute or relative ischemia; it has been used with some regularity intra-operatively during neurovascular surgery. From 1960 to 1989, there were case reports and series of over 120 severe HI patients treated with systemic cooling. From these, the only conclusion that could be drawn is that toxicity—coagulopathy, arrhythmias, and so forth — is low at temperatures >30°C for <72 hours.
Several laboratory and clinical investigations have shown that hypothermia can decrease excitatory amino acid and free radical production and lower the brain’s metabolic demand or cerebral metabolic rate of oxygen consumption (CMRO2) thereby reducing anaerobic glycolysis and lactate production.16,17 In vivo physiological studies have shown that the optimum temperature for maintenance of normal intracranial and cerebral perfusion pressure is 34.4 to 35.5°C.18
To evaluate the use of hypothermia clinically in the treatment of severe HI, a MEDLINE search from 1960 to 2004 was undertaken, utilizing the key terms cooling, head injury, and hypothermia. One hundred forty-two articles were identified, of which five were randomized clinical trials (Table 5–2).
In the early 1990s, coincident with an intense effort to identify pharmacological inhibitors of the secondary injury cascade, clinical studies were developed to investigate the potential effects of mild and moderate hypothermia on outcome after severe HI. The studies organized by Clifton et al19 and Marion et al20 proceeded almost simultaneously, albeit independently.
In the Clifton et al study,19 46 patients were either randomly assigned to hypothermia as an adjunct to standard ICP control management (n = 22) or maintained normothermic with active temperature control efforts (n = 24). Patients were excluded if cooling could not be initiated within 6 hours of injury. Target temperatures were 37°C in the normothermia group and 32 to 33°C in the hypothermia group, where active cooling was maintained for 48 hours. The dichotomized Glasgow Outcome Score (GOS) at 3 months—good recovery/moderate disability (GR/MD) versus severe disability/vegetative/dead (SD/V/D) was not statistically significant (p = 0.287) but strongly favored improved neurological out-come in the hypothermia group—GR/MD 52 versus 36%.
| Reference | Evidence Class | Description | Conclusion |
|---|---|---|---|
| Clifton et al19 | I | 46 patients from a single center with severe HI (GCS) were randomized to a standardized medical management protocol with hypothermia (32–33°C for 48 hours) or standardized medical management with maintenance of normothermia (37°C). | Based on GOS, patients treated with hypothermia had improved outcomes— GR/MD 52% versus 36%; SD/V/D 47% versus 63%. Even though there was a 16% absolute increase in good outcome in the hypothermia group statistical significance was not achieved. |
| Marion et al20 | I | 40 consecutive patients from a single center were randomly assigned to treatment with hypothermia (32–33°C for 24 hours) or were kept normothermic 37–38°C) with both groups receiving maximal standard ICP management. | The hypothermia group had significantly lower ICP (p < 0.004); however, although there was a trend toward improved neurological recovery at 3 months— 60% of patients in the hypothermia group with GR/MD versus 40% in normothermia group—statistical significance was not reached (p < 0.24). |
| Shiozaki et al23 | I | 33 patients whose ICP remained above 20 mm Hg 5–6 hours after induction of high-dose barbiturate therapy were randomly assigned to a hypothermia group (core temperature 33.5–34.5°C for 48 hours) and a control group. | Mild hypothermia reduced ICP by a mean of 10.4 mm (p < 0.01) and increased the CPP by a mean of 14 mm (p < 0.01). Eight patients in the hypothermia group survived (50%), with five having a good recovery at 6 months, whereas only three in the control group survived (18%), with only one having a good recovery (p < 0.05). |
| Shiozaki et al24 | I | A multicenter trial of hypothermia in 91 patients whose ICP had been controlled below 25 mm Hg by conventional therapies, including barbiturates. The patients were randomly assigned to hypothermia (33.5–34.5°C for 48 hours) or normothermia. | No difference in clinical outcome was seen at 3 months after injury. There was a statistically significant increased incidence of pneumonia, meningitis, leukocytopenia, thrombocytopenia, hypernatremia, hypokalemia, and hypomagne-saemia in the patients in the hypothermia group. |
| Clifton et al21 | I | A multicenter trial of 392 patients randomized to hypothermia (33°C for 48 hours) or normothermia. | No difference was demonstrated in good outcome and poor outcome, including mortality between study groups (p = 0.79). In patients over 45 years old, there were more poor outcomes in the hypothermia group (88%) compared with the normothermia group (69%, p = 0.08) and in patients hypothermic on presentation (35°C or less) outcome was uniformly poor. |
Abbreviations: CPP, cerebral perfusion pressure; GCS, Glasgow Coma Scale; GOS, Glasgow Outcome Score; GR/MD, good recovery/moderate disability; HI, head injury; ICP, intracranial pressure; SD/V/D, severe disability/vegetative/dead.
Marion et al20 in a similar study of 40 patients found equally encouraging physiological and outcome results. In the 20 patients randomized to hypothermia, ICP was significantly lower than in the normothermia patients (p < 0.004) and the incidence of hourly ICP levels over 20 mm Hg was also significantly lower (p < 0.001). Three months after injury, the GOS was improved in the hypothermia patients—60% versus 40% GR/MD as was the Disability Rating Scale (DRS)—scores of 5 or less in 45 versus 25%. However, in neither measure of outcome were the findings statistically significant (p < 0.24).
The encouraging results in these two studies led to the National Institutes of Health-funded, phase III multicenter clinical trial, National Acute Brain Injury Study: Hypothermia (NABISH). This study had a planned sample size of 500 and was initiated in 1994 in 11 participating centers. The target temperature was 33°C within 8 hours of injury, maintained for 48 hours. Three hundred ninety-three patients were enrolled in NABISH; 193 were randomly assigned to normothermia and 199 to hypothermia. Enrollment was stopped after 5 years when an interim analysis indicated the futility of achieving a statistically significant result at the 500-patient level of study design.21
The GOS at 6 months after injury demonstrated no difference between treatment groups—57% of patients in both groups had a poor outcome (SD/V/D), with a mortality of 28% in the hypothermia group and 27% in the normothermia group. Additional subgroup analyses failed to identify any group benefiting from hypothermia treatment. There were more poor outcomes in patients over 45 years of age in the hypothermia group— 88% versus 69%—and all patients with an admitting temperature <35°C had a worse outcome, irrespective of treatment group.
Initial analysis did not reveal any significant imbalances in patient characteristics at the time of enrollment; however, subsequent review of the study data identified several potential confounding variables.22 There was a significant difference in the number of patients over 45 years of age and the number of patients hypothermic on admission by center. Given the finding previously noted of the poor outcomes in patients >45 years old in the hypothermia group, it was felt that such intercenter variability may have affected the findings. In addition, there was a significant inter-center variability in the incidence of postrandomization hypotension—mean artieral pressure (MAB) p < 70 mm Hg (p < 0.001) and cerebral perfusion pressure (CPP) p < 50 mm Hg (p < 0.05)—both factors that might independently affect outcome.
The study by Shiozaki et al23 in 1993, similar to the early reports of Clifton et al19 and Marion et al,20 found a beneficial effect of hypothermia in a small group of patients. The major difference in these studies was that patients in the Shiozaki et al study were randomized to hypothermia only after failure of barbiturates to control ICP.23 From a physiological standpoint, hypothermia significantly reduced ICP and increased CPP, while reducing mortality. Of the 33 patients entered into this randomized study, eight patients in the hypothermia group (50%) and three (18%) in the control group survived (p < 0.05).
A study by the same authors in 2001 randomized 91 patients whose ICP had been controlled by conventional therapies to mild hypothermia—34°C for 48 hours—or normothermia.24 There was no significant difference in clinical outcome at 3 months.
Three meta-analyses of the use of hypothermia in the management of severe HI have been conducted, all with similar findings: The available evidence from prospective randomized clinical trials at best demonstrates marginal benefit in improving neurological outcome.25–27
Therefore, the currently available evidence in adults does not support the use of induced mild hypothermia to improve outcome after severe HI. It, however, may be an option to control elevated ICP. When used in this fashion, there must be a clear understanding of the physiological and metabolic effects of hypothermia.28 As core temperature is lowered to 33 to 34°C, there is a significant impairment in pulmonary gas exchange due to basal atelectasis with a resultant decrease in arterial oxygen saturation and inspired oxygen concentration (paO2/FiO2) from a normal of 0.400 mm Hg to as low as 0.25 mm Hg. This may increase the risk of pneumonia in patients already at high risk for this complication. Although there is some decrease in cardiac index and some direct effect on cardiac conduction, no significant arrhythmias or cardiovascular instability has been described in clinical studies to date.
Suppression of antidiuretic hormone directly affects kidney function with a resultant cold diuresis and creatine clearance may be reduced by up to 60%, predisposing the patient to potential electrolyte disturbances. Additionally, as the temperature is lowered potassium (K+) is driven intracellularly with resultant hypokalemia. Conversely, during rewarming rebound hyperkalemia occurs. Hypophosphatemia and hypomagnesemia have also been observed.
Although coagulation disturbances are a theoretical concern with hypothermia, they have not been shown to be clinically significant. Immunosuppression, however, does occur with significant depressions in the total leukocyte, absolute neutrophil, and absolute lymphocyte count, with the potential for increasing infection risk.29 Thus, the utilization of hypothermia for ICP management requires the type of clinical vigilance undertaken for barbiturate and other similar therapies.
The issue of therapeutic hypothermia has been addressed in the HI guidelines for infants, children, and adolescents with the following recommendation at the option level: “Despite the lack of clinical data in children, hypothermia may be considered in the setting of refractory intracranial hypertension.”30
Treatment Effectiveness
From a global perspective, the treatment principles contained in Bullock et al’s7 Guidelines for the Management of Severe Brain Injury should offer a unique opportunity to study the impact of guidelines utilization on the effectiveness of current treatment in the improvement, if any, in outcome. The HI guidelines effort began, in part, in response to the significant variability in HI care documented in a 1991 survey of trauma centers.31 It was felt that the generation of treatment parameters based on the best-available scientific evidence and their general acceptance and practice would at the least improve the likelihood of improved neurological outcome. Although the guidelines have been widely disseminated in both print and electronic media not only to neurosurgeons but also to trauma surgeons and critical care specialists, their implementation has been limited. Arecent survey of 824 trauma centers found that ICP monitoring was undertaken in only 33% of patients meeting the guidelines criteria as compared with 26% from the 1991 survey. Overall, only 16% of centers were found to be in “full compliance” with standards or guidelines level of recommendations.2
There has been little research published on the effect on outcome of using protocols based on the guidelines. This is in part due to limitations in study design; in the United States, it is not possible to prospectively study guideline- versus nonguideline-based HI care. Thus, what little research that exists utilizes preguidelines historical controls for comparison. In 1998, Spain et al32 demonstrated a 22% reduction in intensive care unit days, a 24% decrease in ventilator days, and a 20% reduction in survivors’ hospital costs after implementation of the guidelines. No clinical outcome data were provided. In a community hospital setting, Palmer et al33 reported an odds ratio of 3.0 favoring a good outcome in 56 guidelines-managed patients as compared with 37 preguidelines patients. The most comprehensive study was undertaken by Fakhry et al34 allocating patients to three cohorts—preguidelines (n = 219), full guideline compliance in 50% (n = 188), and full guideline compliance in 88% (n = 423). Outcomes were based on both the GOS and the Ranchos Los Amigos Score (RLAS). Over 61% of the patients in the highest full-guidelines compliance group had a GR/MD, compared with 50% in the low-guidelines compliance group and 43% in the controls (p < 0.01). Similar findings were seen in the RLAS (p < 0.04).
An effort was begun in the late 1990s to introduce the HI guidelines to several eastern European countries (Croatia, Hungary, Slovakia, Slovenia), where, because of severely limited resources there existed virtually no treatment for severe HI. This offered the opportunity to improve medical care and to evaluate prospectively whether the guidelines improved the natural history of severe HI. Preliminary data found the 2-week mortality reduced from 47 to 24% and GR/MD at 6 months improved from 18 to 29% after introduction of the guidelines along with the technology needed to implement them, such as ICP monitoring.
It is thus a paradox that it is unlikely that the efficacy of evidence-based guidelines will be proven with the same level of scientific rigor espoused by the guidelines. Does this mean that the guidelines should be abandoned? Common sense and expert opinion would hold otherwise.
Conclusion
In the last 2 decades, substantial scientific evidence regarding the treatment of severe traumatic brain injury has been accumulated through basic research, prospective database research, and randomized clinical trials. Rigorous analysis of this scientific data has allowed the formulation of evidence-based guidelines that improve the care of patients with such injuries. Neurosurgeons and other medical specialists who care for traumatic brain injury should have ready access to these guidelines in their most up-to-date form to apply the best available evidence to the care of their patients. (The guidelines are available at http://www.brain-trauma.org/pdflibrary.nsf/Main/Guidelines/$;File/Management+and+Prognosis+of+Severe+Traumatic+Brain+Injury+Preview.pdf)
References
- Narayan, R. Clinical trials in head injury. J Neurotrauma 2002;19:503–557
- Bullock, R, Chesnut, R, Clifton, G, et al. Part I. Guidelines for the Management of Severe Traumatic Brain Injury, Management and Prognosis of Severe Traumatic Brain Injury. New York, NY: Brain Trauma Foundation; 2000:1–165
- Aarabi, B, Alden, T, Chesnut, R, et al. Management and prognosis of penetrating brain injury. J Trauma 2001;51:S1–S86 (suppl)
- Teasdale, G, Jennett, B. Assessment of coma and impaired consciousness. Lancet 1974;2:81–84
- Teasdale, G, Knill-Jones, R, Van der Sande, J. Observer variability in assessing impaired consciousness and coma. J Neurol Neurosurg Psychiatry 1978;41:603–610
- Menegazzi, J, Davis, E, Sucov, A, Paris, P. Reliability of the Glasgow coma scale when used by emergency physicians and paramedics. J Trauma 1993;34:46–48
- Bullock, R, Chesnut, R, Clifton, G, et al. CT scan features. In: Bullock, R, Chesnut, R, Clifton, G,, et al, eds. Guidelines for the Management and Prognosis of Severe Brain Injury. Part II. Early Indicators of Prognosis in Severe Traumatic Brain Injury. New York: Brain Trauma Foundation; 2000:65–115.
- Gentry, LR, Godersky, JC, Thompson, B. MR imaging of head trauma: review of the distribution and radiopathologic features of traumatic lesions. AJR Am J Roentgenol 1988;150:663–672
- Snow, R, Zimmerman, R, Gandy, SE, et al. Comparison of magnetic resonance imaging and computed tomography in the evaluation of head injury. Neurosurgery 1986;18:45–52
- Kelly, AB, Zimmerman, RD, Snow, RB. Head trauma: comparison of MR and CT—experience in 100 patients. AJNR Am J Neuroradiol 1988;9:699–708
- Gibson, R, Stephenson, G. Aggressive management of closed head trauma: time for reappraisal. Lancet 1989;2:369–372
- Feldman, Z, Contant, C, Robertson, C, et al. Evaluation of the Leeds prognostic score for severe head injury. Lancet 1991;337:1451–1457
- Quigley, M, Vidovich, D, Cantella, D, et al. Defining the limits of survivorship after very severe head injury. J Trauma 1997;42:7–10
- Wesner, M. An evaluation of Think First Saskatchewan: a head and spinal cord injury prevention program. Can J Public Health 2003;94:115–120
- Wright, M, Rivera, F, Ferse, D. Evaluating the Think First head and spinal cord injury prevention program. Inj Prev 1995;1:81–85
- Busto, R, Dietrich, W, Globus, M, et al. The importance of brain temperature in cerebral ischemic injury. Stroke 1989;20:1113–1114
- Chopp, M, Knight, R, Tidwell, C, et al. The metabolic effects of mild hypothermia on global cerebral ischemia and recirculation in the cat: comparison to normothermia and hypothermia. J Cereb Blood Flow Metab 1989;9:141–148
- Tokutomi, T, Morimoto, K, Miyagi, T, et al. Optimal temperature for the management of severe brain injury: effect of hypothermia on intracranial pressure, systemic and intracranial hemodynamics and metabolism. Neurosurgery 2003;52:102–112
- Clifton, G, Allen, S, Barrodale, P, et al. A phase II study of moderate hypothermia in severe brain injury. J Neurotrauma 1993;10:263–273
- Marion, DW, Obrist, W, Carlier, P. The use of therapeutic moderate hypothermia for patients with severe head injuries: a preliminary report. J Neurosurg 1993;79:354–362
- Clifton, G, Miller, E, Choi, S, et al. Lack of effect of induction of hypothermia after acute brain injury. N Engl J Med 2001;344:556–563
- Clifton, G, Choi, S, Miller, E, et al. Intercenter variation in clinical trials of head traumaexperience of the National Acute Brain Injury Study: Hypothermia. J Neurosurg 2001;95:751–755
- Shiozaki, T, Sugimoto, H, Taneda, M. Effect of mild hypothermia on uncontrollable intracranial hypertension after severe head injury. J Neurosurg 1993;79:363–368
- Shiozaki, T, Hayakata, T, Taneda, M, et al. A multicenter prospective randomized controlled trial of the efficacy of mild hypothermia for severely head injured patients with low intracranial pressure. J Neurosurg 2001;94:50–54
- Harris, O, Colford, J, Good, M, Matz, P. The role of hypothermia in the management of severe brain injury: a meta-analysis. Arch Neurol 2002;59:1077–1083
- McIntyre, LA, Fergusson, DA, Hebert, PC, et al. Prolonged therapeutic hypothermia after traumatic brain injury in adults: a systematic review. JAMA 2003;289:2992–2999
- Henderson, W, Dhingra, V, Chittock, DR, et al. Hypothermia in the management of traumatic brain injury: a systematic review and meta-analysis. Intensive Care Med 2003;29:1637–1644
- Metz, C, Holzschuh, M, Bein, T, et al. Moderate hypothermia in patients with severe head injury: cerebral and extracerebral effects. J Neurosurg 1996;85:533–541
- Ishikawa, K, Tanaka, H, Shiozaki, T, et al. Characteristics of infection and leukocyte count in severely head injured patients treated with mild hypothermia. J Trauma 2000;49:912–922
- Adelson, PD, Bratton, SL, Carney, NA, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children and adolescents. J Trauma 2003;54(Suppl):S235–S310
- Ghajar, J, Hariri, R, Narayan, R, et al. Survey of critical care management of comatose, head injured patients in the United States. Crit Care Med 1995;23:560–567
- Spain, D, McIlvoy, L, Fix, S. Effect of a clinical pathway for severe traumatic brain injury on resource utilization. J Trauma 1998;45:101–105
- Palmer, S, Bader, M, Qureshi, A, et al. The impact on outcomes in a community hospital setting of using the AANS traumatic brain injury guidelines. J Trauma 2001;50:657–664
- Fakhry, SM, Trask, AL, Waller, MA, Watts, DD. Management of brain-injured patients by an evidence-based medicine protocol improves outcomes and decreases hospital charges. J Trauma 2004;56:492–499
- Bullock, R, Chesnut, R, Clifton, G, et al. Part I. Guidelines for the Management of Severe Traumatic Brain Injury, Management and Prognosis of Severe Traumatic Brain Injury. New York, NY: Brain Trauma Foundation; 2000:1–165
< div class='tao-gold-member'>