Central pain pathways in Parkinson’s disease. Left: The lateral system is formed by the neospinothalamic, the neotrigeminothalamic, the cervical bundle and the beam of the dorsal horn whose fibers terminate in the lateral thalamus, the primary and secondary somatosensory areas, the parietal operculum and the insula. Right: The medial system is constituted mainly by the paleospinothalamic, spinomesencephalic, spinoreticular, spinoparabrachial hypothalamic and spinothalamic tract fibers, which terminate in the parabrachial nucleus, the locus coeruleus, the peri-aqueductal gray substance, intralaminar and medial thalamic nuclei, thalamic ventral caudal parvocellular nucleus and ventral caudal portae, the insula, parietal operculum, the secondary somatosensory cortex, the amygdala and the hippocampus.
Paleospinothalamic, spinomesencephalic, spinoreticular, spinoparabrachial hypothalamic and spinothalamic tract fibers comprise the medial system. This system participates in the cognitive–evaluative and affective aspects of pain, pain memory and autonomic responses. Even in the presymptomatic stage of PD, before substantia nigra pars compacta neuronal degeneration is appreciable, several structures within the medial pain pathway are the site of neuronal loss and Lewy body development [16].
The neospinothalamic, neotrigeminothalamic, cervical bundle and beam of the dorsal horn comprise the lateral system, which functions in the sensory discriminative component of pain, informing pain localization and duration [16, 17].
In PD patients, significant dropout of free nerve endings and Meissner’s corpuscle has been demonstrated, independent of age or disease duration [18]. It is postulated that these changes, which comprise a component of deafferentation, contribute to significant sensory dysfunction in PD.
Degenerative changes occurring in the spinal cord, particularly in lamina I of the dorsal horn, have been described early in PD pathogenesis [19]. The serotonergic (including raphe nuclei), noradrenergic (including locus coeruleus) and dopaminergic descending pathway networks originating in the cerebral and brain stem structures participate in the modulation and integration of pain signals processed in the dorsal horn. There is also evidence for glutaminergic and GABAergic involvement [20]. Collectively, these networks up- or downregulate pain gain thresholds [21].
Conceivably, the multifocal neurodegenerative processes in PD may cause damage to many of these pertinent structures participating in nociceptive information relay, modulation, reception and interpretation anywhere along the continuum from peripheral receptors to cerebral centers.
Other data demonstrate that nigrostriatal denervation results in the inhibition of the lateral thalamic region, with consequential reduction in the sensory discriminative dimensions of pain and the potential development of a central pain syndrome [17]. Evidence also suggests that striatum involvement in PD produces disturbance in the emotional registration of pain, as well as the perception of pain intensity [22]. It has been shown that pain occurs more frequently in untreated patient’s during their “off” periods, which correlates with low dopaminergic activity in the striatum [22]. Accordingly, dopamine appears to confer an analgesic role through mechanisms in multiple supraspinal structures including the basal ganglia, insula, anterior cingulate cortex, thalamus and periaqueductal gray matter [23]. Not surprisingly, lowered pain thresholds were shown to be normalized after administration of levodopa (l-DOPA), with findings corroborated by positron emission tomography imaging [24]. Of note, other studies have produced less supportive and sometimes conflicting evidence for the effects of dopaminergic activity on pain [25, 26].
Risk factors for pain in Parkinson’s disease
Age
This is a somewhat controversial area but there is emerging data suggesting that PD-related pain (especially dystonic pain) is associated with a younger age of onset [7, 27, 28]. Other data suggest no significant association between these two variables [4, 6, 29].
Gender
Studies indicate that mechanical sensitivity is higher in women with PD [30], who may have a predilection to musculoskeletal pain, including neck and lower back pain [4, 31]. Other data appear to dispel significant gender differences [7, 27–29, 32].
Disease severity and duration
Some data have supported a significant relationship between PD disease duration, severity (often reflected by motor symptoms) and the presence of pain [28, 29, 33]. Such findings have not been disclosed by other studies [4, 7, 32].
Depression
In one study that evaluated depression in PD patients with and without pain, the incidence of depression was the same but there was a correlation between pain intensity and the presence of depression [34]. Depression was also more severe in PD patients with pain, compared with those without. Similar correlations have also been seen in a few other studies, but have not been definitely demonstrated in others [29, 32]. The recognition of depression in PD has practical implications since the mood disorder negatively impacts on pain processing and other experiential dimensions but is generally amenable to treatment.
Preexisting comorbidities
The notion that at least some pain in PD is due to nonneurological, age-related changes has been supported in the literature [4, 7] This may be explained in large part by the natural progression of preexisting degenerative musculoskeletal conditions such as osteoarthritis (both appendicular and axial joints), as well as other chronic painful conditions often associated with advancing age (e.g. peripheral neuropathy).
Pain classification and treatment
Pain in PD has been categorized in several ways; however, grouping of sensory and pain symptoms into “primary” (germane to the nervous system) and “secondary” (for all etiologies extrinsic to the nervous system) represents a basic approach [35]. Nociceptive pain can be regarded as pain arising from actual or potential injury to nonneural tissue and is a result of activation of nociceptors [5]. In the PD patient population, neuropathic pain may be defined as pain caused by lesions of the central or peripheral somatosensory system leading to central or peripheral neuropathic pain, respectively. In this scenario, symptoms are thought to arise, at least in part, from altered pain processing [5].
Similarly, pain and sensory symptoms may be broadly attributable to PD (directly) or non-PD causes. There is growing acceptance of a more practical classification scheme that identifies the following categories: musculoskeletal pain, radicular/neuropathic pain, dystonia-related pain, akathitic discomfort/pain and central parkinsonian pain [27, 36]. Pain management options can be usefully arranged according to these groupings (see Table 16.1). For many PD patients, pain symptoms may fall into more than one category [4, 37].
Etiological classification of principal pain syndromes in Parkinson’s disease and their practical management
| Etiological category | Painful syndromes | Management |
|---|---|---|
| Primary pain | Central pain | Dopaminergic therapy (levodopa, dopamine agonists) Anti-inflammatory agents, opioids, antiepileptics, tricyclic, antidepressants and atypical neuroleptics |
| Secondary pain | Musculoskeletal pain | Musculoskeletal examination, eventually rheumatological/orthopedic evaluation Physical therapy and occupational therapy Medical therapy: dopaminergic therapy (for parkinsonian rigidity and akinesia); anti-inflammatory and analgesic drugs (for rheumatological and orthopedic conditions) Surgical therapy: orthopedic joint surgery if indicated |
| Radicular/neuropathic pain | Neurological examination, eventually electrophysiological and imaging investigations Physical and occupational therapy Medical therapy: antidepressants, anticonvulsants, opioid analgesics, nonsteroidal anti-inflammatory drugs, also in combination Surgical therapy: decompressive surgery if indicated | |
| Dystonia-related pain | Evaluation of painful dystonia and its relationship to dopaminergic medication: provide more continuous dopaminergic stimulation Additional medical therapy: anticholinergics, amantadine, injections of botulinum toxin, baclofen | |
| Pain related to akathisia | Dopaminergic therapy (levodopa, dopamine agonists), opioids, clozapine | |
| Pain related to restless legs syndrome | Lifestyle changes and activities: decreased use of caffeine, alcohol and tobacco Eventual supplements to correct deficiencies in iron, folate and magnesium A program of moderate exercise and massaging the legs Dopaminergic therapy (dopamine agonists, levodopa); benzodiazepines, opioids, anticonvulsants |
Musculoskeletal pain
Musculoskeletal pain is reported among one-half to two-thirds of PD patients [28, 39]. Symptoms are referable to muscular, truncal and appendicular articular/periarticular discomfort including aching and cramping sensations, occurring in the conspicuous absence of definite dystonia. There may be manifestations of preexisting or coexisting rheumatological and orthopedic conditions, such as osteoarthritis or spinal degenerative disease [28]. Pathophysiological biomechanics in PD including rigidity and reduced joint/limb mobility, as well as abnormal posture and gait, have been regarded as major contributing factors [38, 40, 41]. Joints commonly affected by pain include the shoulders, hips, knees and ankles, whereas muscle tightness and cramps are typical complaints in the arm, calf, neck and paraspinal muscles [27].
Musculoskeletal pain treatment is dictated by the specific underlying cause. In instances where parkinsonian rigidity predominates, dopaminergic pharmacotherapy, physical therapy and a customized physical exercise regimen are beneficial. There may be distinct benefits from a passive range of motion exercises aimed at reducing the risk of contractures seen with decreased mobility [36].
Orthopedic and rheumatological comorbidity, which may be exacerbated by uncontrolled PD motor symptoms, is often best managed with the input of the appropriate specialist. There is often a role for analgesics in conjunction with physical therapy – these drugs include nonsteroidal anti-inflammatory agents, acetaminophen and low-potency opioids [42]. There may also be a role for serotonergic and noradrenergic antidepressant medications.
When more conservative measures produce suboptimal results, intra-articular corticosteroid injections, nerve blocks and possibly surgical intervention may be exploited.
Dystonic pain
This pain subtype is seen in up to 70% of PD patients [7, 29], and may be among the most painful symptoms a PD patient may experience. The culprit dystonia may be due to PD itself, or may be the adverse effects of therapy with dopaminergic agents or DBS. Dystonia in this context is often focal or regional. The foot is often involved in early-onset PD, with prominent toe cramping, usually early in the morning [43]. With many patients, exacerbations tend also to occur late in the dosing interval when dopaminergic effects are wearing off. Conversely, about one-third of patients on long-term levodopa therapy may have significant dystonia during their “on” periods [44].
For dystonia that is attributable to a decrement in dopaminergic drug effects, several approaches to reduce the “off” period may be beneficial. These include earlier or preemptive dosing of levodopa, using controlled-release formulations, or employing longer-acting dopamine agonists [45]. The severity of early-morning dystonia in some cases may warrant the use of subcutaneous apomorphine, which confers a rapid therapeutic effect. Other dopaminergic strategies to avoid these untoward effects during the “off” periods have utilized continuous infusion of duodenal levodopa [46], intranasal or subcutaneous apomorphine [47], transdermal rotigotine [48] and lisuride infusion [49]. Other pharmacological agents that are not primarily dopaminergic but may be used empirically for “off”-period dystonia include benzodiazepines, baclofen and lithium. Focal dystonia may also be amenable to botulinum toxin injections [50, 51], and further uses in PD patients are being explored in clinical trials.
The nonpharmacological treatment of pain associated with focal dystonia includes DBS of the subthalamic nucleus (STN) [52–54] or globus pallidus internus (GPi) [13] targets. Repetitive transcranial magnetic stimulation is a promising modality in development that is further discussed below.
Peak-dose dystonia requires a different strategy, often necessitating a reduction of the levodopa/dopaminergic agent dose. Dystonic dyskinesias may respond to amantadine [55, 56] or low-dose clozapine [57], and a therapeutic trial of these agents may be worth considering.
Radicular/neuropathic pain
This type of pain is localized to a spinal root or peripheral nerve territory. This pattern of nerve injury may be seen in up to 25% of PD patients with pain [7, 38]. The literature espouses that median neuropathy at the wrist (carpal tunnel syndrome) is seen more commonly in PD patients [58]. A predilection to other entrapment or compression syndromes in PD, such as that involving the radial nerve, has been less robustly supported through case series [59, 60]. In some patients, these neuropathies may represent chronic sequelae of limb immobility and arrested positions while being wheelchair- or bed-bound. The repeated trauma conceivable from the repetitive movement of tremor may be another contributory mechanism. The adverse motor milieu in PD (including rigidity, tremor, akinesia/bradykinesia, dystonia, camptocormia, and festination) is believed to promote structural stresses and maladaptations affecting the spine and appendicular musculoskeletal system that places nerve roots and peripheral nerves at increased risk for compression and/or entrapment. Evaluation of pertinent symptoms should prompt a workup that may include electromyography (EMG) and/or neuromuscular ultrasound, as well as other neuroimaging, which may demonstrate lesions amenable to release/decompression surgery.
Back pain with or without radicular pain is more prevalent in PD patients compared with controls [41]. Data also suggest that radicular pain is more prevalent in PD patients (14–35%) [61] compared with the general population (10%) [41]. Accompanying radicular symptoms may also necessitate electrodiagnostic and neuroimaging evaluation, and findings may inform a decision about spine surgery.
It has been shown that peripheral neuropathy is seen more commonly in PD patients, and various associations/risk factors have been implicated, including the use of amantadine [62], cumulative dose effects of levodopa and low vitamin B12 levels [63, 64].
If pain appears to correlate strongly with “off” periods, then a trial of dopaminergic therapy would be a reasonable endeavor. The pharmacological approaches to management of radicular or neuropathic pain in PD is not very different from that in other patients. Agents include tricyclic antidepressants (e.g. amitriptyline, nortriptyline, desipramine), serotonin and norepinephrine reuptake inhibitors (e.g. venlafaxine, duloxetine), calcium-channel α2δ ligands (e.g. gabapentin and pregabalin), topical lidocaine and opioids (sparingly).
Akathitic discomfort/pain
Akathisia is characterized by a subjective restlessness and uncomfortable urge or impulse to move persistently, producing an intolerance of remaining still. This usually responds to levodopa and other dopaminergic treatments, especially when it occurs prominently in the “off” period [36]. Clozapine may be additionally helpful [65]. Restless legs syndrome (RLS) may also be present in PD patients and potentially represents another source of discomfort. Symptoms tend more commonly to involve the lower limbs and are generally worse at night. Restless legs syndrome appears to be more prevalent among PD patients, at 8–20% [66, 67] compared with 1% [66] in the general population. Treatment for RLS in this context would typically comprise levodopa and/or a dopamine agonist. The use of pramipexole and ropinirole in this context has not been studied in a controlled trial, although they are approved for the treatment of RLS in the general population.
Central pain
Central pain occurs when there is a primary lesion or dysfunction in the central nervous system. It has been reported in 10–12% of PD patients [4, 32]. The poorly localizable pain is typically severe and often has a quality of tingling, burning or itching, or it may have an autonomic or visceral character that usually prompts uninformative investigations. It may be the basis for several bizarre pain manifestations that can be seen in PD involving the face/head, mouth/throat, chest, abdomen, pelvis and genitalia [68].
This pain type is relatively uncommon and, although is not thought to be due to dystonia or rigidity, it tends to coincide with motor fluctuations, being more prominent in “off” periods. On this basis, it is thought to be a consequence of dysfunction in dopaminergic autonomic centers, which may also play a role in pain control [69].
Treatment with dopaminergic agents is usually beneficial. In cases with a poor response to this first-line approach, conventional analgesics, atypical antipsychotics (e.g. clozapine), tricyclic antidepressants and opioids may be beneficial. Opioid use in PD patients should always be exercised with fair caution, given the potential to exacerbate parkinsonism, especially at higher doses. There may be pain improvement utilizing STN-DBS; however, the available evidence on this is sometimes conflicting [54, 70].
Due to interplay between chronic pain and depression, the treatment of any comorbid depression (seen in about 40–50% of PD patients) [71] is quite important in a pain management strategy. Antidepressants with overlap indication for the particular subtype of the pain (e.g. neuropathic) should be used if possible. Psychotherapy may also constitute part of the multimodal therapy that is beneficial in the management of both depression and pain.
Duloxetine, a serotonin and norepinephrine reuptake inhibitor known to exhibit both antidepressant and analgesic properties, was evaluated in an open-label study recruiting PD patients having pain excluding that associated with dystonia, limb rigidity, nocturnal muscle spasm or lumbago, as well as non-PD-related pain (e.g. post-stroke central pain) [72]. Duloxetine 60 mg daily for 6 weeks was used, and pain relief was seen in 65% of patients. Interestingly, the pain threshold, depression and quality-of-life measures were not significantly affected. More robustly designed future trials would be helpful in better defining the potential benefits of duloxetine for pain in PD patients.
Choosing an analgesic medication with pleiotropic effects may increase the chance of improving health-related quality of life, as opposed to choosing a pain medication without other actions.
Nonpharmacological management
To more comprehensively address pain in PD, a multidisciplinary approach employing medications as well as nonpharmacological management modalities is required.
Physical therapy and exercise programs that may be beneficial include aerobic exercises, systematic stretching, and strength and balance training. Relevant cautionary measures should be taken, given the inherently increased fall risk in the PD patient population. Exercise in this context has been shown to improve physical functioning, leg strength, balance, gait and overall health-related quality-of-life measures [73]. There is also data that purport a pain improvement benefit, especially with musculoskeletal type pain [74].
Complementary and alternative medicine modalities for pain in PD patients include homeopathy, “traditional medicine,” acupuncture and massage. These options are usually sought when the response to more conventional approaches is suboptimal [75]. Data indicate an effective reduction of stiffness and pain in PD patients receiving massage therapy [76] and acupuncture [77, 78].
The effects of DBS and other stereotactic brain surgeries on the various PD pain subtypes have been evaluated in a few studies [12, 53, 79]. Investigators have shown a significant reduction in musculoskeletal and dystonic-type pain with DBS targeting the bilateral STNs [53, 79]. However, this treatment appeared to be associated with increased risk for postoperative deterioration in radicular/peripheral neuropathic pain and spine disease-related back pain [53]. There have also been reports of significantly reduced PD-related pain in patients receiving unilateral pallidotomy [12].
Prospective analgesic therapies
Repetitive transcranial magnetic stimulation (rTMS) is an emerging modality that has been producing promising results [80, 81]. The precise mechanism of action of rTMS is not fully understood, but data show that, when applied over the primary motor cortex, there is modulation of the activity in pain-processing areas including the thalamus, the insular cortex and the anterior cingulate cortex [82]. There may be downstream involvement of the endogenous opioid system [83].
Cranial electrotherapy stimulation is another noninvasive modality that involves the application of a small electrical current through the head utilizing ear-clip electrodes. Cranial electrotherapy stimulation was shown to produce significant analgesic effects in a randomized, double-blind, placebo-controlled, non-PD sample [84]. The therapy was also investigated in a sample of 13 PD patients with pain [85]. Pain among those enrolled was not necessarily PD related. In this double-blinded study, participants were randomized to either an active or sham device, which they used for 40 min daily over 6 weeks. Although patients assigned to the active-treatment arm had higher PD motor impairment scores and a longer disease duration, they obtained significantly greater pain reduction compared with the sham group. No serious study-related adverse events were reported. Cranial electrotherapy stimulation may represent another nonpharmacological option that confers particular advantage to this patient population, which is already at risk for polypharmacy.
Other neuromuscular complications in Parkinson’s disease
As previously alluded to, various mechanical stressors seen in PD may place patients at significantly increased risk for various peripheral nerve injuries. These stressors include repetitive involuntary motion, increased appendicular and axial tone, relative immobility and contractures. Peripheral nerve injuries may also result from injuries sustained with falls, to which PD patients are particularly susceptible.
There are data describing peripheral nerve injuries in other movement disorders. For example, secondary cervical radiculopathy has been found in up to 32% of patients with cervical dystonia [86]. However, the literature is quite deficient in the description of peripheral nerve injuries among PD patients.
In a study of 1087 PD patients, a diagnosis consistent with peripheral nerve injuries was found in 4.8% [87]. Among those with electrodiagnostic confirmation (42%), radiculopathies, mononeuropathies and peripheral polyneuropathy were seen in equal proportions (41% for each). Most commonly seen were L5–S1 radiculopathies and peripheral polyneuropathy with evidence of active/ongoing denervation, as well as severe median neuropathies at or distal to the wrist (carpal tunnel syndrome).
Entrapment mononeuropathies may be an initiating factor in the development of limb deformities in patients with late-stage parkinsonism [59]. This was illustrated in a case series of four patients with advanced PD demonstrating radial compression neuropathy and flexion deformity of the wrist and hand [59]. The common contributing factor appeared to be advance chronic motor disability with wheelchair or bed confinement. In this scenario, preventative measures include frequent position changes and avoidance of sleeping in wheelchairs. To help prevent the development of a fixed flexion wrist deformity, the use of a wrist splint is also recommended.
More recently, it has been shown that neuropathy is more prevalent in PD patients, and prolonged levodopa therapy as well as vitamin B12 deficiency may be further implicated [64, 88]. One study disclosed peripheral neuropathy in 55% of PD patients and described a correlation between cumulative levodopa exposure and neuropathy severity [64].
Rajabally and Martey found neuropathy in 37.8% of PD patients versus 8.1% in age- and gender-matched controls [63]. Cumulative levodopa exposure correlated with the duration of PD and vitamin B12 levels in PD patients with neuropathy. The precise pathophysiological details have not yet been elucidated, but it is believed that genetically predisposed PD patients may develop levodopa-induced vitamin B12 deficiency with consequential neurotoxic accumulation of methylmalonic acid [Figure 16.2]. On the basis of their findings, the investigators advised that vitamin B12 monitoring and supplementation, as well as serial clinical assessments for neuropathy be conducted in PD patients [89].
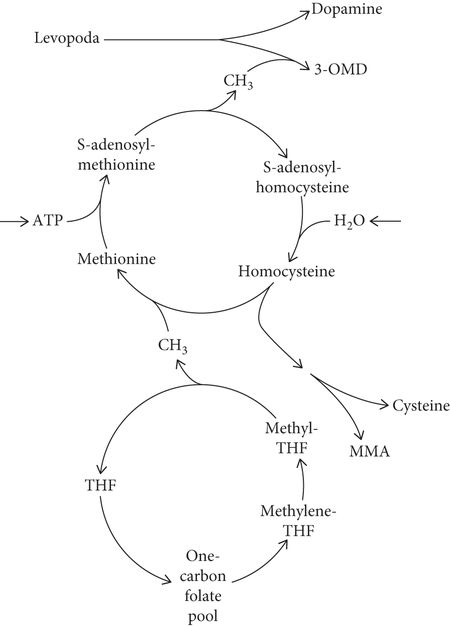
Facets of levodopa and homocysteine metabolism hypothesized to be relevant to peripheral neuropathy in patients with Parkinson’s disease. Conversion of levodopa into 3-O-methyldopa (3-OMD) depletes methyl-group (CH3) reserves and leads to homocysteine production. Subsequent homocysteine remethylation (into methionine) requires vitamin B12 (cobalamin) as a cofactor and obtains its CH3 from the one-carbon folate pool. Involvement of methylenetetrahydrofolate (methylene-THF) in supplying the CH3 makes polymorphism in methylene-THF reductase an important determinant of plasma homocysteine level. Homocysteine trans-sulfuration (into cysteine) requires vitamin B6 (pyridoxine). A pathway leading to methylmalonic acid (MMA) makes MMA as well as homocysteine a marker of functional vitamin B12 deficiency. ATP, adenosine triphosphate.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



