(1 patient at 60)
Electrode configuration
With very few exceptions,37 most clinicians have used unipolar (monopolar) electrode configurations for successful pallidal DBS in primary dystonia (Table 9.1).
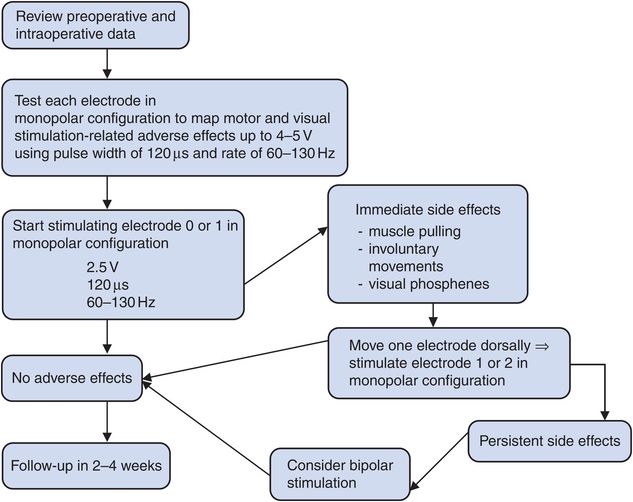
In our experience, the vast majority of DBS patients with dystonia are treated using a unipolar configuration, with single or – in more than half of the cases – multiple adjacent active electrodes. In the remaining patients, electrodes are set in a bipolar or tripolar configuration, a choice usually dictated by intolerable motor side effects occurring when stimulating in unipolar mode.34
Stimulation parameters
Optimal practices for the three basic DBS parameters of stimulation (amplitude, pulse width, and rate) have not been established. As previously mentioned, there is a good level of “educated guess” involved in the initial choice of stimulation settings, because aside from dystonic tremor, no immediate improvement is usually observed at the time of initial DBS programming. In addition, there is no consensus on how long a delayed response should be awaited before consideration is given to changing electrode configuration or parameters of stimulation.38
The early literature concerning stimulation of the GPi documented excellent results using very long pulse widths, fast rates, and high amplitude just below the adverse effect threshold,39,40 although other authors have used parameters more consistent with those used for pallidal stimulation in patients with Parkinson’s disease.41,42 However, use of high stimulation rate (> 100 Hz) and prolonged pulse width more rapidly depletes the battery of primary cell neurostimulators, leading to frequent neurostimulator replacements (as frequently as every 12–24 months). For children and young adults treated with DBS, such frequent surgery to replace neurostimulators poses a significant life-long medical burden. Thus, the use of stimulation parameters that extend neurostimulator battery longevity, or the use of rechargeable neurostimulators with greater battery life, is attractive for these patients.
Amplitude
Amplitude controls the volume of tissue that is affected by the stimulation. The largest experience to date pertains to the use of neurostimulators that provide constant voltage stimulation, allowing delivery of stimulation between 0 and 10.5 V.
In most cases, stimulation-induced adverse effect thresholds (mostly caused by stimulation of the internal capsule) dictate the maximum amplitude; for well-located DBS leads in the posteroventral pallidum or dorsolateral STN, therapeutic amplitudes typically range between 1.0 and 3.5 V (Table 9.1).
Lack of stimulation-related adverse effects above 5.0 V should prompt a re-evaluation of lead position. One factor to keep in mind is the possible impedance variability at the interface between the electrode surface and surrounding tissue, which may affect the proper delivery of therapeutic stimulation by DBS devices used in constant voltage mode. Studies have suggested that impedance variability can occur in chronically implanted DBS electrodes, although the clinical consequence is unclear given the typical stability of therapeutic effects over time for most patients.43 DBS systems delivering a constant current of stimulation have become available more recently, but the benefit of delivering stimulation in this mode remains to be determined.
Pulse width
The pulse width specifies the duration of the electrical pulse used to stimulate the target area. The current required to stimulate a neural element decreases as the pulse width increases. This non-linear relation is described by an inverse exponential function.1 While amplitude controls the volume of stimulated tissue, pulse width seems to determine which neuronal elements are recruited within that volume.44
Initial experience with pallidal DBS for dystonia promoted the idea that long pulse widths are necessary to guarantee optimal clinical outcome, because stimulation had to “suppress” a large number of cell bodies. As theories of DBS mechanisms of action evolve, this practice has been challenged.
Many clinicians now use much shorter pulse widths (Table 9.1), and one experimental study reported comparable results in patients randomized for a period of 10 hours to short (60–90 μs), medium (120–150 μs), and long (450 μs) pulse widths.45
As in Parkinson’s disease, STN stimulation does not seem to require long pulse widths to control dystonic symptoms. The few available case series indicate that pulse widths of 60–120 μs can produce significant improvement.18,22,23
Rate
The rate (frequency) of stimulation, measured in Hertz (Hz) (the number of electrical pulses delivered per second), plays a crucial role in the therapeutic effect of DBS. The beneficial effects of relatively high rates of stimulation have been studied systematically for thalamic and subthalamic stimulation in PD patients.1 The use of higher rates of stimulation was supported also in dystonia by the results of a study showing a superior response of dystonic symptoms to pallidal DBS as stimulation rate was increased from 50 to 250 Hz.46
However, we and others have documented that for treating dystonia, lower rates of stimulation (60–80 Hz) can be as effective as higher frequencies.34,47–49
One of the very first reports describing the successful treatment of dystonia with GPi DBS described maximal symptomatic relief with stimulation at 50 Hz, although at a very high pulse width.50 Nevertheless, lower stimulation rates were abandoned when reports of larger patient series demonstrated marked clinical improvement employing 130 Hz or higher rates and wide pulse widths.4,30,42,51 Importantly, we have found that clinical outcome is not correlated with the total energy delivered, suggesting that positive results in dystonia do not seem to be dependent on the amount of stimulation delivered but on its quality and location.34
When STN is targeted for dystonia, optimal results have been reported with high stimulation rates (130–150 Hz) similar to those used for Parkinson’s disease and tremor.18,22,23 In the STN, stimulation rates of 130 Hz provided better outcomes than 60 Hz in a small cross-over study of seven patients with cranio-cervical dystonia.52
In the absence of clear guidelines, the choice of optimal parameters of stimulation for treating dystonia is ultimately left to individual experience. Based on our experience with GPi DBS, we routinely start stimulation activating electrode 0 or electrode 1 in unipolar configuration. Amplitude is usually set at 2.0–2.5 V, pulse width at 120 μs, and rate at 60 Hz. Figure 9.1 summarizes some of the basic steps of initial DBS programming for dystonia.
Follow-up programming
Similar to initial programming, follow-up visits should use a systematic approach. An initial review of interim changes (including symptom response, medication changes, and adverse events) should be followed by device interrogation and analysis of where the stimulation parameters are within the therapeutic range established during the initial programming visit. Based on this clinical evaluation, a management plan can be formulated to provide stimulation and/or medication adjustments (Figure 9.2).
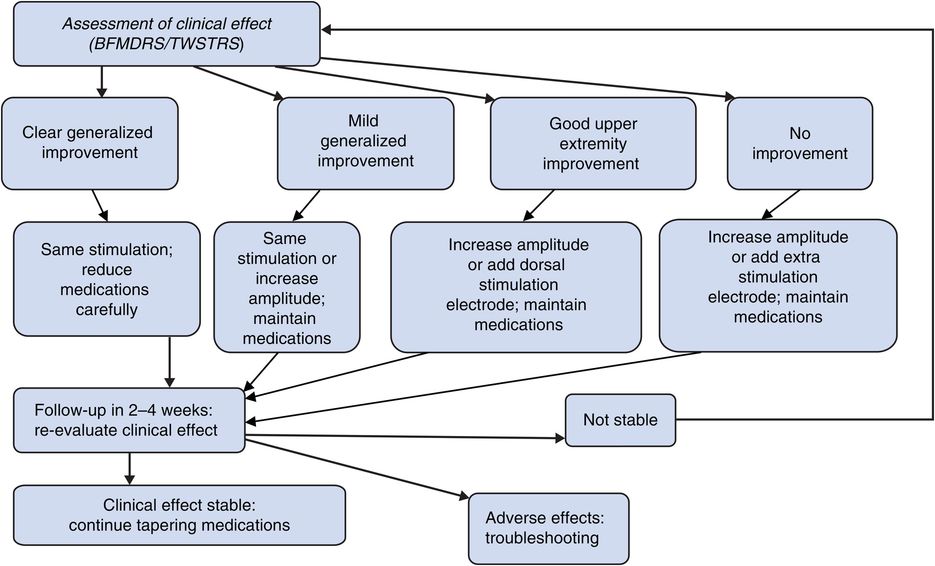
Follow-up deep brain stimulation programming algorithm for dystonia treated with globus pallidus stimulation.
Unless particular adverse effects occur, we usually see patients in follow-up approximately one month after initial programming, an interval long enough to observe clinical responses that may guide DBS adjustments.
When clinical response of GPi stimulation is absent or minimal, stimulation settings can be systematically adjusted by increasing either amplitude (our preferred choice) or pulse width (up to 210 μs). Alternatively, in order to expand the volume of stimulated tissue, we often find it helpful to activate a second or even third electrode in a multi-electrode unipolar configuration. Occasionally, in adult patients, we have also seen additional improvement with slight increases of stimulation rates to 70–90 Hz.53
Most patients with dystonia are able to substantially decrease their medications, which are usually relatively ineffective, once the beneficial effect of stimulation is established, although this may take weeks or months to occur.4,42,54 Medication tapering in dystonic patients with DBS is more an art than a science, and there is no consensus in the literature on this point.38 We normally start tapering medication as soon as we observe some clear sign of clinical improvement, beginning with the drugs that were least effective according to the patient or their caregiver. We discuss and design slow tapering schedules, in which elimination of each medication takes weeks or months to occur. In our experience, more than half of patients with dystonia are able to discontinue all medications after DBS of GPi, while the others are able to reduce their medication requirements by about 60%.34
Troubleshooting
Approaches to troubleshooting are discussed in Chapter 12, but issues relevant to patients with dystonia are presented here. There are several reasons for a suboptimal response to DBS therapy, including unresponsive disease, inaccurate lead placement, and suboptimal DBS programming or medication adjustments. Poorly managed expectations can also generate a subjective perception of failure, even when clinical results are objectively apparent. Finally, initial benefit may give way to symptom exacerbations in the presence of technical failures or device malfunctions.
A detailed clinical assessment before and after surgery should be performed to monitor progress.
The widely used Burke–Fahn–Marsden Dystonia Rating Scale (BFMDRS) (Appendix I), the Unified Dystonia Rating Scale (UDRS), and the Dystonia Study Group (DSG) scale showed a similar and acceptable range of internal consistency and inter-rater reliability.55–58 The Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS) is available for torticollis (Appendix J).59 We also recommend videotaping each clinical examination for future reference and comparison with baseline.
On average, the mean improvement of dystonia scores seems to be lower in secondary dystonias in comparison to primary dystonias, with a larger standard deviation, indicating more variable results. Normal MRI is one possible predictor of a favorable outcome for secondary dystonias.60
Appropriate DBS lead location is absolutely necessary to achieve optimal results. No amount of expert DBS programming can compensate for a poorly placed lead. Suboptimally located DBS leads normally result in unacceptable stimulation-induced adverse effects at low levels of stimulation or produce no effect (beneficial or adverse) with test stimulation at high amplitude and pulse width. The nature and localizing value of the adverse effects signaling an inadequate lead location are summarized in Tables 9.2 and 9.3. Imaging studies should be obtained in these cases, in order to determine as accurately as possible the location of the DBS lead and for planning revision.
| Effect | DBS lead is likely | Structure stimulated |
|---|---|---|
| Dysarthria at low levels of stimulation | Too posteromedial | Corticobulbar fibers |
| Tonic muscle contraction at low levels of stimulation | Too posteromedial | Corticospinal fibers |
| Visual phenomena | Too deep | Optic tract |
| No effect at high levels of stimulation | Too superior, anterior, or lateral | – |
| Effect | DBS lead is likely | Structure stimulated |
|---|---|---|
| Dysarthria and facial pulling | Too anterolateral | Corticobulbar fibers |
| Tonic muscle contraction at low levels of stimulation | Too anterolateral | Corticospinal fibers |
| Diplopia | Too ventromedial | Nerve root of oculomotor nerve |
| Mood and behavioral disturbances | Too ventromedial | Ventromedial portion of STN |
| Nausea, discomfort, gait ataxia | Too posteromedial | Red nucleus |
| Paresthesia | Too posterior | Medial lemniscus |
| Akinesia, depression | Too deep | Substantia nigra |
Adequate DBS device programming is crucial for the overall success of DBS. Ideally, patients treated with DBS should receive programming at the same institution, and by the same team that implanted their device. This provides continuity of care and immediate access to information concerning initial programming and lead placement from the operating room. When suboptimal programming parameters are the suspected cause of DBS failure, the history of programming sessions and responses should be reviewed in detail. If this information is not available, a systematic approach, similar to that carried out during initial programming, should be followed (Figures 9.1 and 9.2).
A sudden loss of stimulation efficacy following previous stable symptom control or intermittent side effects suggests a device-related problem. Hardware-related complications generally include lead fracture, extension wire failure, lead migration, skin erosion, foreign body reaction, neurostimulator malfunction, pain, and other less common events.61 The literature regarding diagnosis, prevention, and treatment of hardware-related DBS complications in dystonia is limited. Hardware-related adverse events, including neurostimulator malfunction, have been reported in long-term follow-up studies. The incidence, ranging from 13% to 40%, seems to be lower in more modern series.34,62 A high incidence of slipped and fractured DBS leads was reported in patients with cervical dystonia.63 In our review of 30 cases followed for at least 2 years (and up to 8 years), we calculated a 4.9% complication rate per DBS lead-year, which is at the low end of the 4.3–9.5% range reported for other targets and disease populations.34
Currently available neurostimulators provide features that may help the programmer in distinguishing device-related problems from other forms of therapeutic failure. These include impedance measurement, battery status indication, and activation and usage counters.
Stimulation-related adverse effects
Tables 9.2 and 9.3 describe the most frequent adverse effects related to stimulation of the GPi and STN. In the case of GPi stimulation, if the current spreads ventrally to the optic tract, it will cause phosphenes (described by patients as bright lights or scintillating visual illusions) and occasionally nausea. Visual side effects can be easily avoided by using more dorsal electrodes for stimulation or reducing the amplitude of stimulation.
The presence of phosphenes may also be helpful in identifying the most ventral part of the GPi; in this case the electrode just above the electrode responsible for producing phosphenes should be used.4,61
Electrical current spreading medially or posteriorly into the internal capsule may evoke tonic muscle contraction of contralateral muscles, often associated with dysarthria.40 Occasionally, involuntary movements (facial or limb dyskinesia) may occur when stimulating the most ventral electrodes in the pallidum. In such cases, amplitude can be reduced or, alternatively, more dorsal electrodes used. If unwanted adverse effects are observed at a low amplitude of stimulation, even lower amplitudes of stimulation combined with increased pulse width can be tested in order to increase current density without further spread of the electrical field. Finally, bipolar or tripolar settings can be used.
Similarly, stimulation of the dorsolateral STN adjacent structures may result in specific side effects that allow the programmer to make the necessary adjustments by reducing the voltage delivered, changing to bipolar electrode configuration, or changing the active electrode (see Table 8.2). Inadvertent stimulation of the ventromedial portion, which projects to areas involved in cognitive and emotional behavior, may result in mood disturbance and behavioral side effects. If the DBS lead is placed too laterally or anteriorly, stimulation of the internal capsule fibers may occur, leading to involuntary muscle contractions in the contralateral body; if placed too ventromedially, stimulation of the oculomotor nerve roots will cause diplopia and gaze deviation. If the lead is placed too posteromedially, there may be stimulation of the red nucleus leading to nausea, extreme discomfort, and gait ataxia.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree