Skull x-ray showing DBS leads in an epilepsy patient treated with deep brain stimulation of the anterior nucleus of the thalamus.
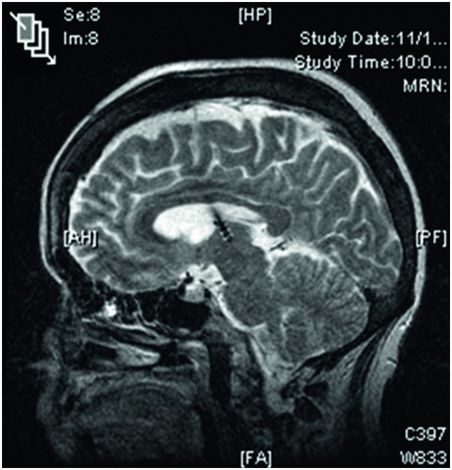
Sagittal MRI image of the brain showing a DBS lead located in the anterior nucleus of the thalamus; note the ability to visualize the four discrete electrodes on the lead.
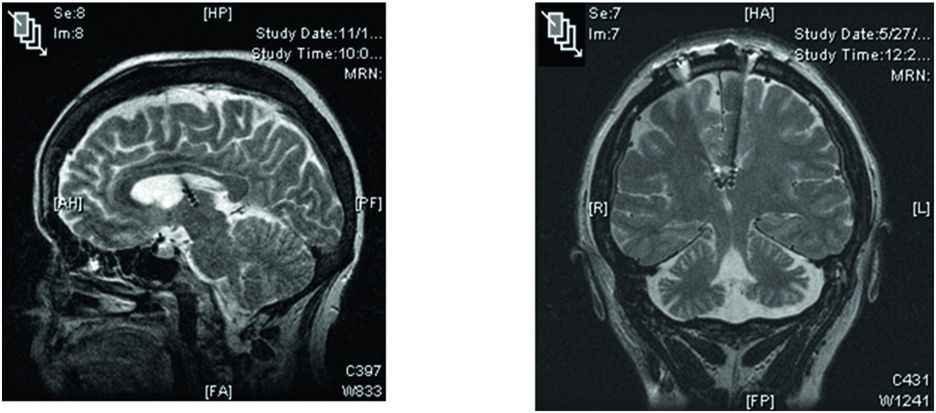
Sagittal and coronal MRI images of the brain showing DBS leads located in the anterior nucleus of the thalamus in patients with refractory epilepsy.
Fisher et al.10 reported that the median seizure frequency decline from the baseline at the end of the blinded phase was 40.4% in the stimulated group and 14.5% in the control group. Furthermore, the most severe seizures were significantly reduced by stimulation. Neuropsychological test scores for cognition and mood did not differ between control and stimulated groups at the end of the blinded phase. Medications remained unchanged during the 3-month blinded phase and the unblinded phase.
After the blinded phase all patients received stimulation from month 4 to month 13 in an unblinded phase, and limited stimulation parameter changes were allowed at 7 and 10 months (increase of amplitude to 7.5 V or increase in rate to 185 Hz). Fisher stated that “Changing stimulation parameters to 7.5 V or 185 Hz did not reduce seizures more than initial settings, but these changes were not systematically studied.” During the open-label phase there was a gradual and sustained improvement. Patients with prior implantation of VNS or prior resective epilepsy surgery also improved.
There were no clinically significant hemorrhages, although five hemorrhages were detected incidentally by neuroimaging. Two patients had acute but transient stimulation-associated seizures. The most common device-related adverse events were paresthesias in 18.2% of patients, implant site pain in 10.9%, and implant site infection in 9.1%, all decreased in frequency between years 1 and 2. In 8.2% of patients the leads were initially implanted outside the AN and had to be replaced.
The authors concluded, “This randomized trial shows benefit of anterior thalamic DBS in some epilepsy patients who were refractory to previous treatments.”
A long-term follow-up study of the SANTE cohort demonstrated sustained efficacy and safety, with a 41% median percent reduction in seizure frequency from baseline at 1 year and a 69% reduction at 5 years. In the 5 years of follow-up, 16% of subjects were seizure-free for at least 6 months. Significant improvement in quality of life over baseline was seen at 1 year and at 5 years.14
The mechanism of action of DBS remains unclear; DBS causes a mixture of excitatory and inhibitory effects, which may disrupt pathological hypersynchronous neuronal networks.
Practical aspects of anterior nucleus thalamic DBS for epilepsy
Initial stimulation parameters for stimulation directed at the anterior nucleus of the thalamus are presented in Table 10.1. Note that unlike in DBS for movement disorders and psychiatric disorders, in which stimulation is delivered on a continuous basis, DBS for epilepsy uses a cyclical paradigm. The approach to programming DBS for epilepsy is detailed in Table 10.2. Note the reliance on imaging to inform selection of active electrode(s).
| Parameter | Typical initial value |
|---|---|
| Amplitude | 5 V |
| Pulse width | 90 μs |
| Rate | 145 Hz |
| Electrode configuration | Unipolar mode: Single electrode or two adjacent electrodes negative, case positive |
| Cycle of cycling | Cycling mode ON: 1 minute on, 5 minutes off |
1. Obtain/review postoperative image to confirm lead location within the ANT
2. Verify system integrity (check electrode impedances)
3. Program initial stimulation parameters (Table 10.1)
4. From postoperative imaging, identify the electrode(s) within the ANT and program as the negative electrode in the unipolar mode (with neurostimulator case programmed to be positive)
5. Verify tolerability of stimulation
6. Program the neurostimulator for patient control
7. Provide the patient and caregiver with instructions on use of patient programmer and tracking of seizures (count, type, severity)
8. Emphasize adherence to antiepileptic drug regimen
Hippocampal deep brain stimulation
Velasco et al.15 studied the effects of subacute electrical stimulation of the hippocampus (SAHCS) in 10 patients with intractable temporal lobe seizures. Bilateral depth hippocampal or unilateral subdural basotemporal electrodes were implanted in the 10 patients for a diagnosis of the site and extent of the seizure focus before a temporal lobectomy. The AEDs were discontinued for 48–72 hours before the continuous SAHCS, which was performed daily for 2–3 weeks. The stimulation parameters were biphasic wave pulses (130 Hz, 450 µs pulse width). The effects of SAHCS on the number of clinical seizures and interictal spikes were determined daily. In seven patients SAHCS “abolished clinical seizures and significantly decreased the number of interictal spikes at the focus after 5–6 days.” The best responses were found by stimulating either the anterior pes hippocampus close to the amygdala or the anterior parahippocampal gyrus close to the entorhinal cortex. Patients underwent an en-bloc temporal lobectomy, and “no evident histopathological differences were found between the stimulated and nonstimulated hippocampal tissue.” The authors concluded that “SAHCS appears to be a safe procedure that can suppress temporal epileptogenesis, with no damage to the stimulated tissue.”15
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







