Effect on dyskinesia is variable (may be pro-dyskinetic in some and anti-dyskinetic in other patients)
Ventral electrodes: greater anti-dyskinetic effect and can block beneficial effects of levodopa on bradykinesia and gait
Posteriorly or medially to internal capsule: tonic muscle contraction
Posteromedially to medial lemniscus: paresthesia. Inferomedially to oculomotor nucleus or its fascicles: diplopia
Laterally to internal capsule: tonic contraction
Posteriorly to primary thalamic somatosensory nucleus (Vc): paresthesia
Notes: ADL, activities of daily living; CM/Pf, centromedian/parafascicularis; DBS, deep brain stimulation; GPi, globus pallidus internus; SNpr, substantia nigra pars reticulata; STN, subthalamic nucleus; Vim, ventral intermediate; Zi, zona incerta.
DBS lead placement in the centromedian/parafascicularis (CM/Pf) nucleus of the thalamus or anterior to the Vim (i.e., in the region of the Voa/ Vop nuclei), however, may suppress levodopa-induced dyskinesia.10 Disability as measured by the Unified Parkinson’s Disease Rating Scale (UPDRS) is not improved with thalamic stimulation because most disability in PD stems from bradykinesia and gait disorders. However, manual tasks worsened by tremor, such as handwriting, may be improved.
A dramatic and beneficial effect of both GPi and STN DBS has been consistently observed. Both interventions when applied bilaterally result in significant improvements in quality of life and in motor fluctuation and dyskinesia.
An improvement in overall motor function and increased “on” time has been seen with both targets. A large multicenter study reported that the average time during the day with good mobility and no dyskinesia increased from 25–30% at baseline to around 75% with STN DBS.11 As a result, quality of life is consistently improved. Nonmotor fluctuation can also respond to STN DBS, with the most notable improvements in asthenia, irritability, and drenching sweats.12 Improved quality and increased total sleep time, as well as decreased daytime sleepiness (likely due to increased nocturnal mobility), have also been reported.13 Pain may also be reduced following DBS, and increased thresholds for pain have been shown with STN DBS.14 Parkinson’s disease-associated camptocormia (characterized by marked flexion of the trunk) often responds poorly to pharmacological treatment. Several reports document sustained improvement of PD-associated camptocormia with bilateral GPi or STN DBS, but the results are mixed.15
Behavioral symptoms can be highly affected by social factors, and social adaptation to the beneficial effects of DBS can be difficult for some patients and their families. Patients who were formerly very disabled can become independent and lose their “sick role,” while their caregivers’ identity is also challenged. Marital relationships can be strained by these changes, and we have observed several patients elect to divorce their previous caregiver and spouse in light of their newly found independence. Working with a social worker and/or psychiatrist can be helpful during this transition.
The degree of improvement obtained with levodopa (usually the first morning dose of anti-parkinsonian medication with or without an additional 50–100 mg of levodopa, see Chapter 2) after overnight withdrawal of anti-parkinsonian medication is highly predictive of the response to STN DBS and probably also to GPi DBS.16 This is an important part of the examination and helps to determine the degree of benefit obtainable with DBS, reinforcing realistic expectations to patients and families.
With the exception of tremor, signs that are not improved with levodopa usually fail to improve with DBS; these include cognitive and psychiatric problems, on-period freezing, and levodopa-refractory dysarthria, dysphagia, and postural instability.16
Choice of stimulation location
GPi and STN DBS can each be applied successfully with relatively similar beneficial effects.
The most profound difference between STN and GPi DBS outcome is the greater reduction of medication achieved with STN DBS. GPi DBS directly suppresses dyskinesia but less medication reduction is generally possible, whereas STN DBS predominantly has an indirect anti-dyskinetic effect achieved through medication reduction. In our experience, STN DBS may also have a greater anti-tremor effect compared to GPi DBS, although not all studies support this. Levodopa is typically reduced by 30–50% with STN stimulation, as opposed to 10–20% reductions with GPi stimulation. Some clinicians report that up to 10% of all patients treated with bilateral STN DBS are able to stop all drug therapy, at least for some period of time.17 Therefore, STN DBS may be appropriate in selected patients who are unable to tolerate adequate doses of anti-parkinsonian medication (because of somnolence, severe gastrointestinal adverse effects, or psychiatric adverse effects in the absence of cognitive impairment), as this treatment typically treats cardinal motor symptoms without the need for high levels of anti-parkinsonian medication. There is some controversy about the role of STN DBS to treat patients with coexistent impulse control disorders (ICD) or dopamine dysregulation syndrome (DDS). Postoperative reduction in dopaminergic therapy has been shown to resolve such behavioral disorders, but sometimes at the risk of postoperative apathy. However, excessive stimulation of the limbic portion of the STN may induce ICD-like behaviors. Benefit on comorbid obsessive–compulsive disorder (OCD) in PD patients has also been achieved with bilateral STN DBS targeting the medial portion of the STN.18 Bilateral STN DBS has a greater propensity (although still small) to cause a variety of behavioral adverse effects, including mania and depression, not usually seen with GPi DBS, especially in patients with pre-existing psychiatric problems. STN DBS may have a greater likelihood of producing mild cognitive adverse effects than unilateral GPi DBS (see below). As a result, GPi DBS might be a better target in patients thought to have a greater chance of psychiatric or cognitive worsening with DBS surgery.
STN DBS may lead to greater neurostimulator battery longevity compared to GPi DBS due to the lower stimulation parameters often required. This results in fewer device replacement surgeries and therefore less cost. The STN may be an easier surgical target because it is more clearly identified by MRI. Despite these advantages compared with GPi DBS, STN DBS may require more frequent follow-up visits, with complex postoperative management of medication and stimulation-induced adverse effects.
Unilateral GPi or unilateral STN DBS has significant beneficial effects (although substantially less than bilateral DBS) and may expose patients to less upfront risk than that of a bilateral procedure (a second DBS lead may later be added when symptoms progress or if the effects of unilateral stimulation are inadequate). We and others have found that optimally applying unilateral STN DBS in many PD patients may be difficult because of the need to reduce anti-parkinsonian medication in order to reduce dyskinesia on the side of the body contralateral to stimulation. This medication reduction may result in relative under-treatment and worsening of parkinsonism on the side of the body ipsilateral to stimulation. On the other hand, we have had excellent results and no difficulty with medication management using unilateral STN DBS in patients with highly asymmetrical, medication-refractory, tremor-dominant PD. Likely due to the difficulties with medication management, unselected patients undergoing unilateral DBS may be more likely to require contralateral surgery with STN as opposed to GPi implantation.
There is ongoing controversy as to the best overall management strategy when applying DBS to a broad population of PD patients. One large randomized trial performed by the US Department of Veterans Affairs found motor benefits of GPi and STN DBS to be similar after 36 months.19 These findings are contradicted by a similar large-scale randomized trial in the Netherlands, which reported a greater improvement in off-state motor symptoms and disability with STN DBS as compared to GPi DBS.20 The most prudent strategy for target selection is to create a customized plan for each individual patient that takes into account the patient’s motor and nonmotor symptoms, degree of asymmetry, and psychosocial state.
Preoperative patient management
For patients undergoing awake surgery, interventions that maximize off-period parkinsonism and reduce the chance of intraoperative confusion may be valuable, as they may facilitate detection of improvement in parkinsonian signs during intraoperative DBS test stimulation and improve the validity of patient-reported adverse effects. Preoperative reduction in anti-parkinsonian medication can achieve both these goals. Surgery conducted completely under anesthesia, on the other hand, does not require preoperative drug modifications.
Levodopa and catechol-O-methyltransferase (COMT) inhibitors should be withdrawn for 12 hours prior to and during awake surgery.
If patients are on high doses of dopamine agonists, it is often helpful to discontinue these 24–48 hours before surgery because of their long half-life. Some clinicians prefer to reduce dopamine agonists one month or more prior to STN DBS surgery to eliminate the effects of these drugs completely. Lastly, other anti-parkinsonian medications that have a greater tendency to cause confusion, such as anticholinergics and amantadine, may be tapered prior to surgery to eliminate their contribution to adverse cognitive effects.
General postoperative patient management issues
Elderly patients sometimes develop postoperative confusion following bilateral DBS lead implantation that may last from a day up to a few weeks. In general, only supportive therapy is required, and anti-parkinsonian medication can usually be reinstituted during this period – although typically at a reduced dose compared to preoperatively. In rare cases of prolonged confusion accompanied by psychotic features (such as hallucinations), the use of atypical antipsychotic agents, such as clozapine, may be useful. Postoperative confusion has been reported less frequently with GPi lead implantation compared to STN surgery, suggesting that this adverse effect might be somewhat specific to STN surgery. If the patient is lucid postoperatively, no immediate change in drug dosage is necessary.
STN DBS lead implantation may reduce dyskinesia, possibly due to micro-lesioning of the pallidal outflow fibers (especially the lenticular fasciculus), or more commonly may improve off-period parkinsonism and reduce the threshold for anti-parkinsonian medication to induce dyskinesia (probably as a result of micro-lesioning of the STN itself). In the latter case, it is often necessary to temporarily reduce anti-parkinsonian medication in order to reduce dyskinesia. GPi lead implantation may improve both off-period parkinsonism and dyskinesia as a result of the micro-pallidotomy.
Mild chorea may occasionally occur following STN lead implantation. Generally this subsides within 48 hours; however, in our experience, this may rarely take 1–2 months to resolve. If significant chorea is present, a temporary reduction in levodopa dosage by 50% or more is prudent until the chorea resolves.
We usually allow patients to recover from surgery for 1–2 weeks prior to beginning DBS device programming. This allows wounds to heal and some of the more obvious micro-lesion effects to subside. The total effect of DBS is achieved as a result of the micro-lesion caused by lead implantation (minor effect) and the direct effect of stimulation (major effect). Commonly, the benefit achieved with micro-lesioning is maximal immediately postoperatively and declines over several weeks as the edema surrounding the implanted lead resolves. Nevertheless, some micro-lesion effects may be noticeable for at least six months.8
The role of postoperative imaging
Postoperative CT or MRI scans can be used to determine if the DBS leads have been implanted in an optimal location and to exclude hemorrhage and other surgical complications.21 We routinely perform postoperative stereotactic CT scans with 1-mm slice thickness, and in select cases perform a postoperative MRI as well. We have found that DBS lead electrode localization based on imaging can be a helpful tool in selecting the electrodes that are most likely to be efficacious for STN or GPi stimulation.
In our experience, electrodes located in the dorsolateral portion of the STN are usually most beneficial (in contrast with some reports suggesting that stimulation above the STN is optimal). Electrodes located in the center of the GPi are most commonly selected for chronic stimulation of that target.
We have found that electrodes located nearest to the ideal target are most likely to be effective. Those located more than 4 mm from the ideal target are rarely clinically effective. When patients have received suboptimal clinical benefit from DBS, postoperative imaging can be very useful to document DBS lead location and to assess the need for lead repositioning. It is also an effective tool for documenting migration of the lead over time, although this is a relatively rare occurrence. A change in threshold for stimulation-induced adverse effects suggests that such migration may have occurred.
Software-based image fusion of a postoperative CT or MRI scan with a preoperative MRI scan provides data regarding exact lead location.22 The clarity of the postoperative image of each electrode on the DBS lead can be improved by adjusting the window settings to reduce the artifact. The location of each electrode can be plotted, based on the image and the knowledge of the electrode spacing on the lead. The position of each electrode can be assessed on the co-registered preoperative MRI with respect to being inside or outside of the anatomical target, and the distance from the ideal location based on the preoperative surgical plan.
Clinicians should consult current safety guidelines published by the device manufacturer before performing any imaging study on a patient with implanted DBS leads or a DBS system.
DBS device programming in Parkinson’s disease
DBS device programming usually takes place over an initial programming session and several follow-up sessions. The goal of the initial session is to identify the most effective electrode(s) to maximize benefit and minimize adverse effects. Programming GPi DBS in patients with dystonia is somewhat similar to GPi DBS in PD. Programming thalamic DBS in PD is similar to that of essential tremor, but programming GPi, STN, or PPN leads in PD patients can be a more complex and time-consuming process.
The fundamentals of DBS programming are discussed in Chapter 6, and the reader is invited to review that chapter before moving on to the specific issues pertaining to programming DBS devices in PD patients that will now be discussed.
DBS may operate in either constant voltage mode or in constant current mode, which adapts the voltage to the impedance of current flow. Although many clinicians continue to primarily use constant voltage mode, we prefer to utilize constant current mode, as this eliminates the effect of changes in impedance, which can occur in the weeks following DBS lead implantation – although probably less commonly over the long term. In addition, recent experimental data demonstrate that impedance may change significantly within a few minutes of starting chronic stimulation.23 Note that impedance measurements in implanted DBS electrodes average 1000 Ω, making 1 mA roughly equivalent to 1 V. Pulse width should be kept at a minimum, as increasing this parameter does not significantly improve clinical efficacy but may make it harder to find effective parameters without causing adverse effects. We use a starting pulse width of 60 μs for all patients. Initial rate is typically between 130 and 185 Hz. Increases in rate generally increase clinical efficacy in a stepwise manner, with a ceiling around 185 Hz.
The ability to use interleaved stimulation is a recent development with newer neurostimulators. This approach alternates stimulation between two programs on the DBS lead, allowing, for example, unipolar stimulation of two different electrodes on one lead at independent amplitudes. Interleaved stimulation enables more precise delivery of current than double unipolar stimulation and can often improve efficacy and reduce adverse effects. We prefer to use constant current stimulation and a rate of 125 Hz when using interleaved stimulation.
Assessment and record-keeping of clinical effects
Recording detailed notes about the observations made during DBS device programming is very helpful in allowing a systematic determination of the best stimulation parameters. For each electrode configuration, it is important to record the amplitude threshold for stimulation-induced adverse effects, the nature of the adverse effects (e.g., tonic contraction due to current spread to the corticospinal tract at 4.0 V), a qualitative description of the degree of benefit (e.g., very good benefit for tremor, moderate improvement of arm bradykinesia), and perhaps a cross-reference to the UPDRS motor score.
In patients with tremor, repeated examination of a task that is highly sensitive to tremor for that particular patient (such as writing and drawing a spiral) may be necessary to differentiate between otherwise clinically similar effects achieved by different DBS electrode configurations and stimulation parameters. It may be valuable to note the severity and distribution of signs and symptoms on both sides of the body (not just contralateral to stimulation), as sometimes DBS may produce ipsilateral effects. For patients with PD, a description of the nature, severity, and distribution of off-period dystonia and on-period or stimulation-induced dyskinesia is also important.
Scheduling Vim DBS programming sessions
Patients should withhold anti-parkinsonian medication at least overnight prior to the initial programming session. Usually a concentrated period of about one hour is adequate to determine the most effective anti-tremor settings in the vast majority of patients. The algorithm (Figure 8.1) begins with the assessment of stimulation-induced adverse effects for each electrode on each DBS lead, followed by evaluation of therapeutic efficacy for each electrode. Many programming clinicians, however, perform an initial evaluation of both stimulation-induced adverse effects and efficacy for each electrode in sequence.
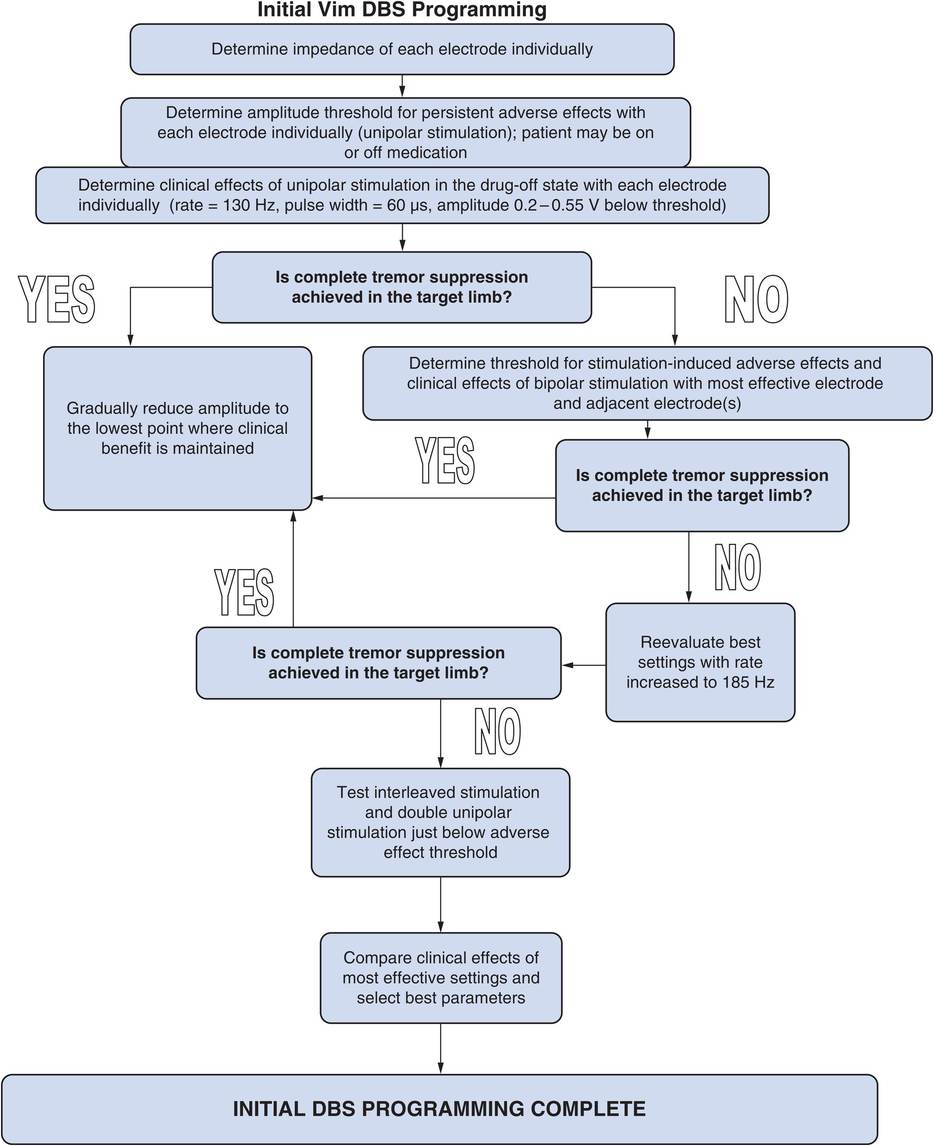
Ventral intermediate deep brain stimulation (Vim DBS) programming algorithm.
Vim DBS programming algorithm (Figure 8.1)
1. Before beginning DBS programming it is useful to record the impedance measurements for each of the four electrodes. Being able to refer to the initial impedances and other electrical properties of the system can be helpful in troubleshooting future hardware problems.
2. If two leads have been implanted, each side should initially be programmed independently, starting with unipolar stimulation.
3. The amplitude threshold for persistent stimulation-induced adverse effects should be determined for each electrode in the unipolar mode. The recommended initial stimulation parameters for Vim DBS are amplitude 0 V, pulse width 60 μs, and rate 130–185 Hz. The amplitude should be gradually increased by 0.5 V every 5–60 seconds (keeping other stimulation parameters constant) while constantly monitoring the patient for subjective and objective adverse effects. An abrupt increase to high amplitude may cause uncomfortable tonic muscle contraction or paresthesia, but tolerance to these adverse effects (especially paresthesia) commonly develops if the amplitude is increased slowly. The amplitude should be increased until persistent adverse effects occur, and then the clinician should note and record the threshold amplitude and nature of the adverse effects.
Analyzing the threshold and nature of the adverse effects with unipolar stimulation allows one to determine the relative location of each of the electrodes to adjacent anatomical structures (Table 8.1).
Determination of efficacy on tremor with Vim DBS
1. First perform a baseline assessment with stimulation off at the beginning of each programming session for comparison and assessment of stimulation benefits.
2. The results of unipolar stimulation provide a base of information that allows one to arrive at bipolar electrode combinations that are more likely to yield good results if it becomes necessary to explore bipolar electrode configurations.
(a) Using unipolar stimulation for each electrode, test the effects of stimulation on tremor at an amplitude 0.2–0.5 V below the threshold for persistent adverse effects. Record the clinical benefit noted for each electrode. The onset and offset of effect on tremor is virtually immediate, so assessments need not be significantly delayed after beginning stimulation with new parameters. If complete tremor suppression can be achieved in the target limb with unipolar stimulation, no further electrode combinations need be tested.
(b) Repeat the above process using the other side (for bilaterally implanted patients).
3. If unipolar stimulation fails to provide adequate tremor control due to stimulation-related adverse effects, then explore logical bipolar electrode configurations based on observations from the unipolar electrode screening. Bipolar stimulation results in more focused effects than unipolar stimulation due to less current spread. Decide which one or two electrodes provide the best anti-tremor effects with unipolar stimulation, and begin bipolar stimulation using these electrodes, determining the amplitude threshold for adverse effects and then efficacy of stimulation.
4. If complete tremor suppression is still not achieved, increase the rate, up to 185 Hz, and re-evaluate efficacy.
5. Test double unipolar stimulation and interleaved stimulation using the best electrode in combination with an adjacent electrode using the same methodology if tremor is not completely suppressed with the single unipolar and bipolar electrode combinations and greater current spread is desired in order to improve efficacy. Many clinicians would explore stimulation using a double unipolar configuration before moving on to a bipolar configuration. With present DBS devices, double unipolar configuration requires constant voltage as opposed to constant current mode. In our experience, interleaved stimulation may be more effective than double unipolar stimulation, because the amplitude used with each electrode can be set individually.
6. Rank in order the electrode configurations that provide the best tremor suppression for each side.
7. Once the best electrode combination has been determined, stimulation parameters can be optimized.
8. At the end of each programming session, send the patient home on the optimal settings thus far determined.
Determination of efficacy on drug-induced dyskinesia with Vim DBS
In patients with PD and levodopa-induced dyskinesia, spread of stimulation current beyond the Vim to the CM/Pf or Voa/Vop regions of the thalamus may block dyskinesia. Verifying the presence or absence of this effect with the anti-tremor stimulation settings in place after levodopa administration is important, since an anti-dyskinetic effect may allow more aggressive drug therapy of off-period bradykinesia and other features of parkinsonism.
Delayed stimulation adjustment and medication adjustment with Vim DBS
Stimulation parameters required to optimally treat motor symptoms become fairly stable by one month postoperatively, and usually only minor adjustments (such as slightly increasing the amplitude) may be necessary beyond three months. Tolerance has not been reported with Vim DBS for PD, but some patients may develop rebound increase in tremor when turning stimulation off at night. As a result, some PD patients must keep stimulation on at night to permit sleep.
Although the majority of patients with PD undergoing Vim DBS require little or no change in medication, patients taking large doses of levodopa to suppress tremor may be able to reduce medication somewhat.
Scheduling GPi, STN, or PPN DBS programming sessions
Programming sessions should generally be no longer than a few hours in duration, as longer sessions often result in patient fatigue and produce variable and unreliable patient performance during tests of bradykinesia.
Most DBS programming in PD patients is performed with patients off medication, and ideally sessions should be scheduled in the morning after overnight drug withdrawal. However, in patients with extremely severe off-period immobility or lack of caregiver support, this may not be easily accomplished without hospitalization and inpatient programming. As a result, a reasonable compromise is to have patients take their first dose of levodopa in the morning to allow them to attend the clinic in a mobile drug-on state; once in the clinic, the effects of anti-parkinsonian medication may be allowed to wear off so that programming can begin in the early afternoon. Patients can then be given levodopa at the conclusion of the session if necessary to assess the effects of stimulation in conjunction with medication before they are sent home. Rare patients may become virtually anarthric and unable to communicate or too disabled to cooperate during programming when completely off medication; such patients may be given just enough anti-parkinsonian medication to induce a suboptimal “on” response without dyskinesia and allow participation. Also in such patients, greater emphasis can be placed on the response of rigidity to various DBS settings, because testing rigidity requires less active patient cooperation and improves much more quickly than bradykinesia with efficacious stimulation parameters.
Functional differences between dorsal and ventral globus pallidus stimulation have been reported (Figure 8.2).24,25 Dorsal stimulation of the globus pallidus produces a marked anti-parkinsonian effect and may induce rather than suppress dyskinesia (similar to STN stimulation); these effects may actually be due to stimulation of the globus pallidus externus (GPe).24 Ventral simulation may worsen akinesia and block the beneficial effects of levodopa on bradykinesia and gait, while improving rigidity and markedly suppressing dyskinesia. As a result, the middle of the pallidum is probably the optimal site for globus pallidus stimulation, taking advantage of these two divergent effects.
Assessment of the effects of DBS on drug-induced dyskinesia is often best performed in the afternoon, once patients have taken more than one dose of levodopa prior to attending the clinic, as dyskinesia commonly exhibits a diurnal pattern (mild in the morning, with worsening in the afternoon and evening).
For patients with GPi or PPN DBS, usually only minor changes in parameters are needed thereafter over the next few weeks to months with infrequent follow-up appointments. In contrast, patients with STN DBS commonly need more frequent follow-up visits, as medication is gradually reduced and stimulation increased, in some cases over many months.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







