Figure 17.1. Respiratory problems in brain-injured patients. Respiratory complications may produce secondary brain injury because of hypoxemia and hypoventilation, promoting vicious circles which jeopardize patient outcome.
ALI = acute lung injury; ARDS = acute respiratory distress syndrome.
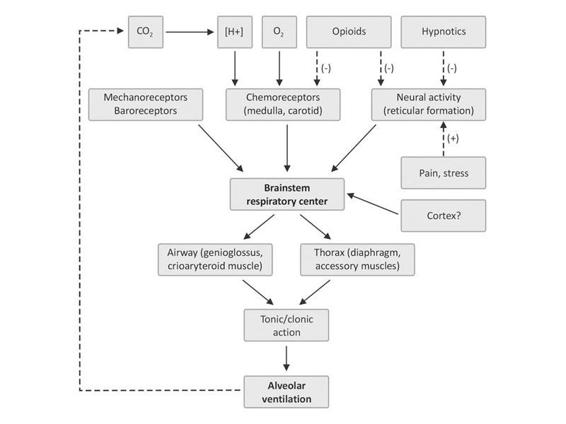
Figure 17.2. Diagram illustrating respiratory centre function.
Respiratory depression is a clinical syndrome characterized by a dysfunction of the respiratory centre. Its diagnosis requires a variable but constant altered level of consciousness and breathing abnormalities (Table 17.1). The most common clinical consequences of respiratory depression are apnoea, bradypnoea or periodic breathing abnormalities, and the inability to maintain a patent airway (tongue falling backwards, retention of secretions and hypotonia of pharyngeal muscles), which may result in alveolar hypoventilation. Also, the decreased functional residual capacity may favour the development of atelectasis and hypoxemia (Figure 17.3).
Diagnosis |
|
Clinical implications |
|
Table 17.1. Diagnosis and clinical implications of respiratory depression.
FRC = functional residual capacity; ICP = intracranial pressure.
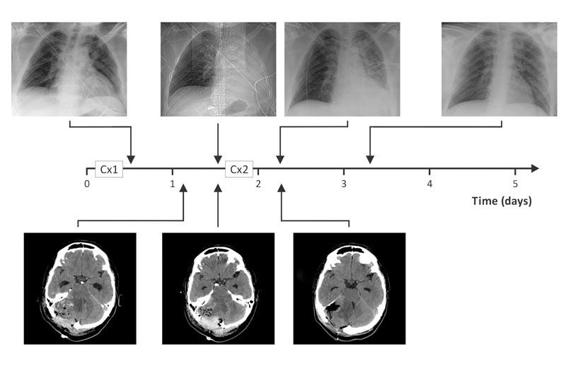
Figure 17.3. A clinical case of respiratory depression.A 29-year-old patient underwent surgery for removal of a tumour of the posterior fossa (Cx1). On the first postoperative day, the patient was drowsy and the CT scan showed edema and partial collapse of the IV ventricle. The patient was carefully observed, but later that day he had to be intubated because of coma. A new CT scan revealed greater collapse of the IV ventricle and postsurgical edema. Total atelectasis of the left lung because of loss of muscular tone, decreased residual lung capacity and decreased cough, was secondary to respiratory depression. The patient was returned to the OR for decompressive surgery (Cx2) and ventilated with positive pressure ventilation and PEEP. A postoperative CT scan showed an expanded IV ventricle. The clinical course was favourable and the patient was extubated two days later.
Respiratory depression may be a serious complication in brain-injured patients because hypoxemia and hypercapnia may induce secondary injury (Figure 17.1). Therefore, airway management should be controlled early in patients with progressive drowsiness and coma. And since cyanosis and tachycardia develop late following hypoxemia, permanent observation of the patient’s level of consciousness and breathing pattern are far more important than preventive measures.
Respiratory depression may also favour the aspiration of gastric contents (Figure 17.1). Patients with trauma and intracranial hypertension may present delayed gastric emptying. It follows that any patient with head trauma or stroke should be treated as a patient with a full stomach at high risk for aspiration. Any sign of vomiting in an unconscious patient is highly suspicious for gastric aspiration. Clinics of aspiration depend on the quality and amount of aspirated liquid, the aspiration of bilious contents or blood being less toxic than acid or food contents. In any case, the chance of secondary infection is high and should prompt antibiotic therapy.
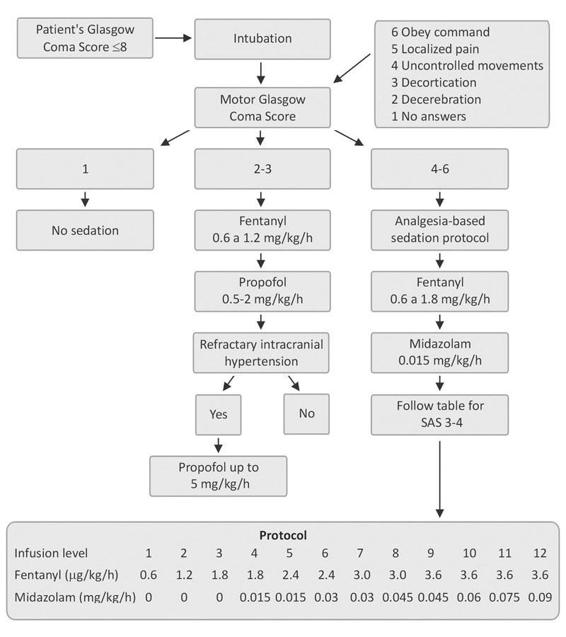
Figure 17.4. A sedation scheme for brain-injured patients in use at the Centro de Pacientes Críticos, Universidad Católica de Chile [Riker, 1999].
SAS = sedation agitation scale.
17.3 Sedation
Sedation in brain-injured patients remains controversial because although it favours mechanical ventilation, it also hampers the evaluation of cognitive function, which is the basic and objective monitor of CNS functioning. However, beyond the objective of facilitating mechanical ventilation, sedation may decrease oxygen metabolism and intracranial hypertension, and control seizures.
There is no clinical study that evaluates the impact of mechanical ventilation, ventilatory modes or sedation in patients with acute neurologic diseases. Guidelines suggest sedative and analgesic drugs based on their pharmacologic effect on ICP, CBF, or CMRO2, more than on data derived from prospective clinical studies. The choice of drugs and doses when ventilating patients with brain injury is extremely heterogeneous.
Sedation in patients with trauma or stroke should be based on: the necessity of clinical evaluation; oxygen metabolism; intracranial pressure; cerebral blood flow and perfusion pressure among others. The protocol for sedation and analgesia in patients with brain injury (Figure 17.4) at our intensive care unit (ICU) involves:
- Use of opioids as first-line agents.
- Use of an hypnotic agent, usually midazolam or propofol.
- Clinical evaluation of sedation according to the sedation against agitation scale (SAS) and the Glasgow motor scale.
- Intracranial pressure and CPP monitoring (if available).
Goal-oriented monitoring of depth of sedation and periodic adjustment are essential to prevent over- and undersedation, which may affect major outcomes and costs. Oversedation hampers neurologic clinical assessment, increases the risk of hypotension (especially when using propofol and barbiturates), increases mechanical ventilation and ICU and hospital length of stay, and may increase nosocomial infections. Undersedation may increase metabolism, oxygen consumption and intracranial hypertension.
The use of muscle relaxants, widely recommended for preventing fighting in patients with intracranial hypertension, is reserved for patients with severe forms of ARDS, with high sedative requirements or for non-conventional modes of mechanical ventilation.
In non-neurologic patients, sedation protocols have been shown to decrease ICU and hospital length of stay and costs. In neurological critically ill patients, a sedation protocol may also decrease infections and secondary hypoxemia and so improve such major outcomes as long-term cognitive functions.
17.4 Hyperventilation
Modulation of PaCO2 has been used for more than 40 years to control intracranial pressure (ICP). The decrease in PaCO2 levels induces a drop in hydrogen ion concentration (increased pH) in the cerebrospinal fluid (CSF), inducing cerebral vasoconstriction and decreasing cerebral blood volume and ICP.
Despite being highly effective to decrease ICP, hyperventilation is not widely accepted as it may induce a decrease in cerebral blood flow (CBF), a change that lasts longer than the decrease in ICP (Table 17.2). There is also evidence that hyperventilation may exacerbate ischemia in hypoperfused zones of the brain. The only randomized prospective study on hyperventilation was performed by Muizelaar et al. in 113 patients with head trauma. Patients ventilated with hypocapnia (PaCO2 25±2 mmHg) had a worse outcome at 3 and 6 months that those treated with eucapnia (PaCO2 35±2 mmHg). So far, there are no data to show that hyperventilation improves long-term outcome in patients with head trauma or stroke.
Regional |
|
Systemic |
|
Table 17.2. Adverse effects (real or potential) of hyperventilation.
CBF = cerebral blood flow; ICP = intracranial pressure; PbO2 = brain tissue oxygenation; SjO2 = oxygen jugular saturation.
We usually try to maintain normo- or light hypocapnia (PaCO2 35-40 mmHg) in our patients, reserving hyperventilation as a second-line, transient therapy for patients with elevated ICP refractory to hypertonic therapy and with normal CPP (Table 17.4). If hyperventilation needs to be tried, brain tissue oxygen partial pressure (PbO2) or jugular venous oxygen (SjvO2) measurements are recommended to monitor the effect of ventilation on CBF and cerebral metabolic rate of oxygen (CMRO2). Although CBF is not routinely measured at the bedside, SjvO2 and PbO2 may help tailor ventilation (Table 17.3). SjvO2 <55% or PbO2 <15 mmHg suggests increased oxygen extraction, decreased CBF or ischemia. In this setting, hyperventilation may be deleterious and treatment should be oriented to increase oxygen delivery (inotropes or vasopressors), decrease oxygen consumption (sedatives) or decrease brain edema (hypertonic therapy). SjvO2 >65-70% suggests hyperemia, so hyperventilation may be tried as a temporary measure to decrease ICP.
Level I (standard) |
|
Level II (guideline) |
|
Level III (options) |
|
Table 17.3. Brain Trauma Foundation recommendations on hyperventilation in brain trauma. Modified from [1].
Mode | Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|



