Fig. 16.1
Subacute combined degeneration of the spinal cord (SCDSC) caused by vitamin B12-deficiency with a clinical history of numbness after 6 months, abdominal sensory level, whereas sense for pain and temperature were normal, no paresis. T2 WI sag. (a) and axial (b–d) showing a hyperintense signal conversion through the entire thoracal part of the dorsal columns, but not cervically
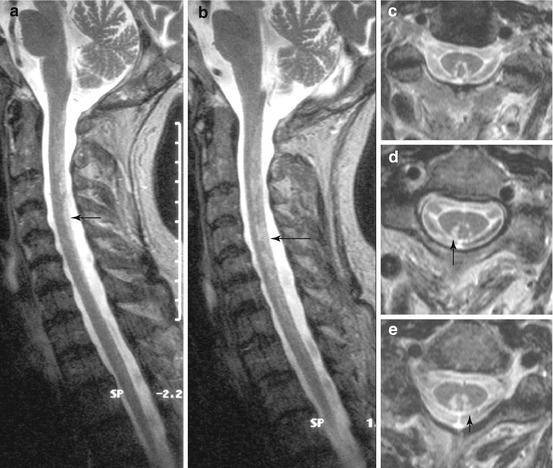
Fig. 16.2
Subacute combined degeneration of the spinal cord (SCDSC) caused by vitamin B12-deficiency in the early course. Sagittal (a, b) and axial (c–e) T2 WI showing patchy hyperintense signal changes in the dorsal part of the spinal cord, most closely in between gracile and cuneate tract, which were shown to be reversible. Arrows, for comarison: Fig. 14.2
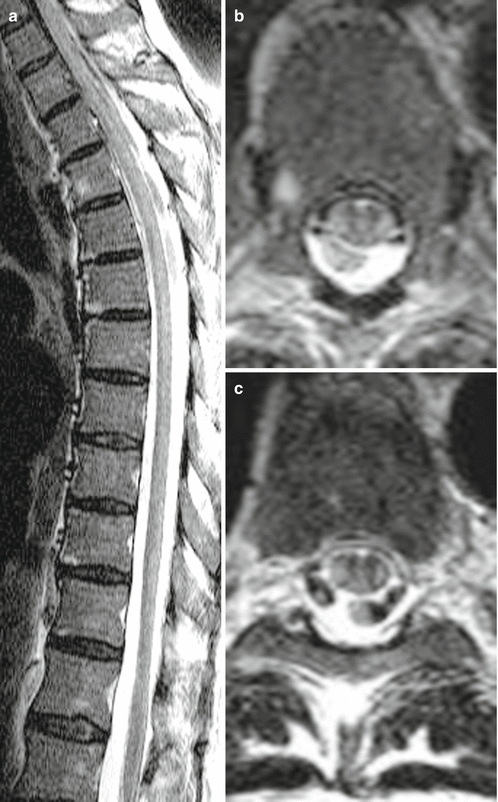
Fig. 16.3
Subacute combined degeneration of the spinal cord (SCDSC) caused by vitamin B12-deficiency in a 63-year-old woman suffering from severe atacic paraparesis with positive pyramidal signs over 9 months; gastrectomy 25 years ago. Sagittal (a) and axial (b–e) T2 WI disclosing hyperintense signal changes in the thoracic lateral and in addition in the lumbal posterior columns (see difference to Figs. 16.1 and 16.2)
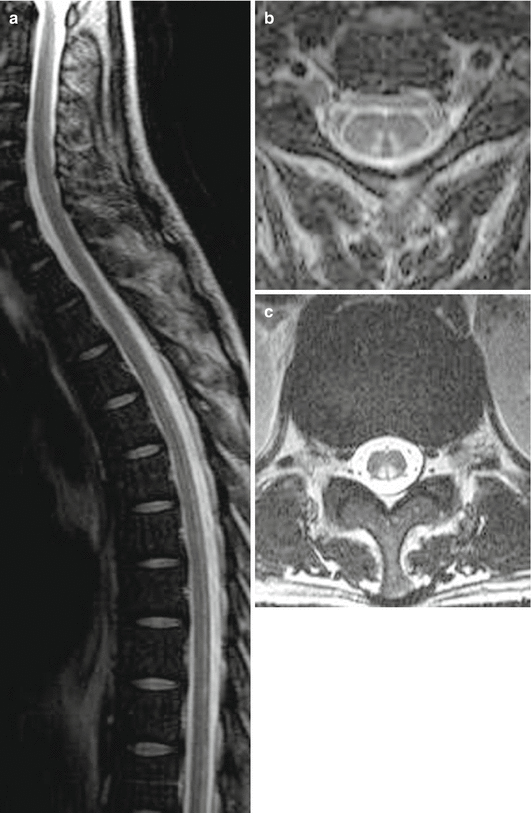
Fig. 16.4
Subacute combined degeneration of the spinal cord (SCDSC) caused by copper deficiency. Extension of hyperintense signal changes on T2 WI in the posterior columns through the whole length of the spinal cord (a, sag.), cervically (b, ax.) not in the course of lumbar fibres (c, ax.); compare with Fig. 16.7
16.2.4 Vitamin B12 Deficiency
Absorption of vitamin B12 requires a glycoprotein (intrinsic factor) produced by the parietal cells in the gastric fundus. Atrophic gastritis, the autoimmune destruction of parietal cells and inactivation of intrinsic factor, is the most common cause of deficiency, leading to pernicious anaemia and SCDSC. Neuropathy, encephalopathy and white matter lesions, and less often optic nerve atrophy, may be associated. Clinically, SCDSC is characterized by symmetric dysaesthesia, disturbance of the position sense and spastic signs, sometimes arising within weeks. In principle, there seem to be two different enzymes of major importance to discuss regarding the pathogenesis of the nervous system disease, both requiring vitamin B12 as a coenzyme [3]: methylmalonic-coenzyme A (CoA) mutase and methionine synthase. Methylmalonyl-CoA mutase catalyses the isomerization of methylmalonyl-CoA to succinyl-CoA, with methylmalonyl-CoA formed from beta oxidation of fatty acids and catabolism of some amino acids. Methionine synthase helps to catalyse the conversion of homocysteine to methionine. Methionine is then converted to S-adenosylmethionine (SAM) which is important in the methylation of myelin basic protein and myelin lipids. Since folate is also integrally involved in the latter pathway, and folate deficiency causes megaloblastic anaemia but not SCDSC, it was assumed that a dysfunction of the first pathway mentioned is causative in vitamin B12 deficiency. However, findings from an animal model provided evidence of the relevance of methionine synthase: N2O irreversibly oxidizes the active cobalt in methionine synthase, rendering the enzyme inactive. In several species, as well as in humans, this may cause SCDSC [3]. Low or unrecognized vitamin B12 deficiency may become evident with N2O anaesthesia or the abuse of the agent. Moreover, prolonged N2O exposure lowers vitamin B12 serum levels for as yet unclear reasons. An entirely new model, independent of vitamin B12 coenzyme functions, considers the pathogenetic role of cytokines and growth factors to explain vitamin B12 deficiency myelopathy [3] (see Sect. 16.5).
16.2.5 Copper Deficiency
Several reports in recent years have revealed that copper deficiency can cause a myelopathy which is clinically and radiologically indistinguishable from SCDSC [4] (Fig. 16.4). Copper is a component of numerous metalloenzymes and proteins which have a key role in maintaining the structure and function of the nervous system. Some of the risk factors for vitamin B12 deficiency, such as malabsorption and previous upper gastrointestinal surgery, are similar. Moreover, an important risk factor for copper deficiency is zinc overload. Zinc interferes with intestinal copper absorption by up-regulating intestinal synthesis of metallothionein, which has a greater affinity for copper. Therefore, the copper bound by the enterocytes is eliminated by their sloughing off into the intestinal lumen. In their review, Jaiser and Winston [4] argued that the phenotypic parallels between vitamin B12 and copper deficiency could be explained by dysfunction of the methylation cycle. The above-mentioned methionine synthase and S-adenosylhomocysteine hydrolase may depend on copper.
16.3 Paraneoplastic Tractopathy
As pointed out by Jacob and Weinshenker [5], the investigation of patients with subacute, evolving and possibly ambiguous symptoms may lead to images which bear a similarity to SCDSC.
Imaging. Symmetric T2 signal abnormalities throughout the thoracic spinal cord seem to be restricted to the corticospinal tract. To our knowledge, there are case reports replicating this finding, but none with neuropathological confirmation. Other than in SCDSC, the tracts showed contrast medium enhancement.
This finding should prompt the search for paraneoplastic antibodies and related cancer (see this review).
16.4 Vitamin E Deficiency
16.4.1 Pathogenesis
Primarily degeneration of axons
16.4.2 Pattern
Posterior columns, most marked in the fasciculus gracilis
Thoracic course of the tract, sparing the lumbar intumescence
16.4.3 Neurological Signs and Symptoms
A combination of spinocerebellar syndrome and polyneuropathy, possibly with pyramidal signs.
Neuropathologic findings of vitamin E deficiency differ substantially when compared to SCDSC. Irrespective of the kind of primary metabolic events in pathogenesis (which probably concern cellular membranes and subcellular organelles), vitamin E deficiency results first in the degeneration of axons, not in a lesion of the myelin sheath as do the above-mentioned disorders [2].
Imaging. Vitamin E deficiency results in an axonal neuropathy involving the centrally directed fibres of the “pseudounipolar” sensory neurons located in the spinal ganglia. The large-calibre fibres are predominantly affected, leading to changes in the posterior columns, most marked in the fasciculus gracilis (Fig. 16.5), extending from the gracile nucleus to the thoracic course of the tract, sparing the lumbar intumescence [6].
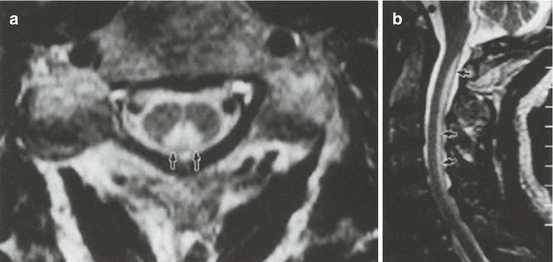

Fig. 16.5
Acquired vitamin E deficiency with axonal degeneration. Axial (a) and sag. (b) T2 WI showing increased signal intensity in the posterior cervical columns (arrows) (From: Vorgerd et al. [6], Springer, Berlin, with kind permission)
Despite several acquired circumstances and hereditary conditions leading to vitamin E deficiency, the case report cited is the only one publishing imaging results.
16.5 Vacuolar Myelopathy
16.5.1 Pathogenesis
HIV-infection, non-AIDS immuno-compromised patients
16.5.2 Pattern
SCDSC-like
Spinal cord atrophy, mainly thoracal
16.5.3 Neurological Signs and Symptoms
Subacutely evolving, painless myelopathy occurring late, mostly in the setting of known infection; see SCDSC.
Vacuolar myelopathy (VM) best known from HIV-infection is mostly looked at as an inflammatory disease. However, from the very beginning there have been discussions on the relationship of vacuolar myelopathy and SCDSC based on a similar lesion pattern and histopathology [7]. Rarely, but repeatedly, VM is observed in non-AIDS immuno-compromised patients, e.g. after steroid medication [7, 8]. The course of the disorders may differ: VM in the case of HIV-infection occurs late and may remain asymptomatic [8], myelopathy as the presenting syndrome of HIV-infection is exceptional [9]. SCDSC with vitamin B12 deficiency, on the other hand, is often heralded by paraesthesia. As supposed by Stacpoole et al. [8], suppression and dysregulation of the immune system may be causative, leading to an impaired repair involving the vitamin B12-dependent transmethylation pathway. S-Adenosylmethionine (SAM; see Sect. 16.1.1), a central component of that pathway, is found to be decreased in AIDS-associated myelopathy. Alternatively, an unidentified infective agent still remains under discussion [8].
In treating the pathogenesis of SCDSC, Hathout and El-Saden [3] commented on the work of Scalabrino and his colleagues [10], which could also be of importance for the development of VM. In the gastrectomized rat, they found sound evidence that vitamin B12 deficiency leads to an imbalance between myelinotoxic and myelinotrophic cytokines and growth factors. Thus, one can speculate that it is dysregulation of the immune system which represents the crucial common part of pathogenesis for VM and SCDSC. A marked macrophage activation and cytokine release was shown in VM as well, and with highly active antiretroviral therapy a decline in incidence has been observed [11].
Imaging. The MRI finding most often observed in patients with the clinical diagnosis of AIDS-associated myelopathy is spinal cord atrophy.
In the series of Chong et al. [12], which was not neuropathologically correlated, 15 of 21 patients showed atrophy, typically involving the thoracic part, with or without association of the cervical segment [12], while 3 of 21 showed no abnormality. Neuropathological studies revealed that the dorsal and lateral white matter columns are mainly affected. The isolated cervical lesion in a rare case of VM with neuropathological confirmation [7], representing a non-enhancing, abnormality without mass effect and moderate T2 hyperintensity, corresponds exactly to SCDSC, restricted to the posterior columns. Enhancement strongly supports HIV-myelitis (Goh et al. [13]; see also Sect. 15.2.6) if lymphoma and the numerous opportunistic infections are ruled out [7]. VM is a diagnosis of exclusion and, as detailed, a categorization as an “immune-mediated” disease one could be justified as well. If any lesion seems to be confined to tracts, brain imaging should be considered in order not to overlook concomitant cerebral pathologies. T2-signal prolongation is caused by every alteration of tissue texture leading to increased water content, or by gliosis, and, thus, vacuolation, axonal loss and Wallerian degeneration all look the same. Nevertheless, knowledge of those underlying pathologies may be of some help for interpretation (Table 16.1).
Table 16.1




Lesions of individual columns – hints for differential diagnosis
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








