Fig. 13.1
Images of a breast cancer patient with multiple metastatic lesions. (a–d) Preoperative axial T1-weighted, contrast-enhanced MRIs demonstrating posterior fossa lesions and multiple supratentorial lesions. This patient had mild ventriculomegaly on imaging but severe nausea and vomiting requiring placement of ventriculoperitoneal shunt before further treatment. (e–h) Axial T1-weighted MRIs with gadolinium enhancement obtained at 6-month follow-up after patient underwent ventriculoperitoneal shunt placement and WBRT
The most common overall presentation of patients with SBM includes a worsening, ipsilateral cranial nerve deficit, or craniofacial pain, depending on the extent and location of the lesion. Physicians and neurosurgeons should have a high index of suspicion for SBM in a patient with known metastatic disease presenting with progressive cranial nerve deficits or facial pain. Greenberg et al. [11], and others since, have described as many as five clinically distinct syndromes in patients that occur at different frequencies: orbital, parasellar, middle fossa, jugular foramen, and occipital condyle syndromes. Middle fossa syndrome predominated (35%) in one cohort of 43 patients [11], whereas parasellar and sellar syndromes predominated (29%) in the meta-analysis by Laigle-Donadey et al. [17], although up to 33% of patients in that review had an undefined clinical syndrome. In this chapter, we will focus our discussion on the two syndromes stereotypically affecting patients with posterior fossa SBM: the jugular foramen syndrome and the occipital condyle syndrome.
The jugular foramen syndrome is characterized by a lesion compressing cranial nerves IX, X, XI, and occasionally XII, depending on the size and exact location of the tumor. Patients may describe this as a dull ache behind the ear or in the occipital region. The clinical picture of these patients often reveals dysphagia and weakness of the palate, hoarseness, and weakness of the ipsilateral sternocleidomastoid and trapezius muscles. Horner’s syndrome has been reported [20], as has Collet-Sicard syndrome [11, 21, 22]. Jugular venous or transverse sinus compression may result in increased intracranial pressure and papilledema [11, 21, 22].
The occipital condyle syndrome is defined by unilateral, often superficial, pain in the occipital region. If the pain initially occurs in the absence of neurological deficits, it often progresses within a short period of time to dysphagia and dysarthria. Unilateral cranial nerve XII palsy is also often present, and patients often complain of a stiff neck [22–24] and pain exacerbated upon flexion or contralateral rotation. The extent of cranial nerve involvement and occipital and neck pain is undoubtedly a function of size and location of the offending tumor.
Diagnosis and Selection for Surgical Intervention
Increased surveillance imaging using computed tomography (CT) and magnetic resonance imaging (MRI) of patients with known cancer has allowed an increase in the detection of asymptomatic intracranial lesions [25, 26]. However, a large number (~30%) of patients who are ultimately found to have brain metastases have had no previous diagnosis of cancer [27]. In these patients, a thorough workup is mandatory during treatment planning and should include extensive imaging, using CT or MRI of the chest, abdomen, pelvis, and even radionucleotide bone scans and positron emission tomography scans. For the neurosurgeon, MRI with and without contrast is the modality of choice and is mandatory before surgical planning, unless it cannot be obtained because of an MRI contraindication. Metastases to the brain are typically found at the “gray-white” junction and have abnormal vascular permeability. Therefore, extensive vasogenic edema is common, and administration of contrast agent typically produces enhancement of the metastases of interest, as nearly all metastases disrupt the blood–brain tumor barrier. Patterns of MRI signal intensity are currently not robust enough to use for diagnosis of specific metastatic tumors, yet many important distinctions can be made. Most brain metastases will show high T2 signal intensity [28]. Necrotic and cystic lesions may demonstrate sharp lesional demarcation but can have variable T1 and T2 signals. T1 rim enhancement is often noted [29]. Hemorrhagic metastases may have heterogeneous enhancement patterns and incomplete rim enhancement and can cause difficulty in diagnostic imaging. Identification of the tumor body is usually sufficient to diagnose a hemorrhaging metastasis versus an arteriovenous malformation or parenchymal hematoma [30]. Notably, MRI offers superior sensitivity to contrast-enhanced CT for detection of metastases in the posterior fossa as well as other small metastases throughout the brain [31].
Imaging studies obtained for a patient with suspected SBM include standard T1- and T2-weighted MRI with and without contrast. It may be helpful to rely on fat-suppressed images in the context of gadolinium administration to pick out enhancing lesions near bone and soft tissue junctions [17]. Skull base CT scan bone windows may provide confirmatory imaging or may substitute for MRI only when absolutely necessary. Radionuclide bone scans or positron emission tomography may be helpful in diagnosis of bony SBM (Fig. 13.2). Laboratory tests should include cerebrospinal fluid cytology to exclude meningeal carcinomatosis [32].


Fig. 13.2
(a, b) Axial CT images of right occipital condyle lesion initially thought to be a paraganglioma but confirmed a metastatic lung adenocarcinoma by needle biopsy. (c, d) T1-weighted axial MRI with and without gadolinium enhancement. (e) Positron emission tomography image consistent with active tumor. (f, g) Coronal T1-weighted MRI with and without gadolinium enhancement. (h) FLAIR imaging. (i) T2-weighted axial MRI. (j) Radiosurgical isodose treatment planning lines for this lesion treated with stereotactic radiosurgery
The number and location of metastases, as well as the overall clinical picture of each patient, must be used to guide the decision to intervene surgically. Single or solitary lesions, >3 cm in diameter, and close to the surface of the brain are most accessible to the neurosurgeon. This is especially true in patients with a large posterior fossa lesion causing mass effect, fourth ventricular compromise, and neurological symptoms (Fig. 13.4). In patients with stable, or relatively stable, metastatic disease, removal of these lesions surgically has been shown to provide substantial survival benefits [33]. Alternatively, having more than three or four small metastases in eloquent brain or in a patient with rampant extracranial disease would be a relative contraindication for surgical intervention, and WBRT or SRS coupled with chemotherapy may be considered. In cases of asymptomatic patients with lesions in the posterior fossa, SRS is an excellent treatment choice (Figs. 13.3 and 13.4). This approach is even more attractive if multiple metastatic lesions are present in both the supratentorial and the infratentorial compartments in asymptomatic patients (Fig. 13.5). Even tumors thought to be moderately or highly “radioresistant” (i.e., melanoma, renal cell carcinoma, thyroid, non-small-cell lung cancer, colon) may benefit from SRS when WBRT might otherwise not prove to be efficacious. There is good evidence to suggest that the mechanism of SRS-induced cell death may be different than that of WBRT [34]. This is discussed later in this chapter. Nevertheless, SRS has been shown to be equivalent to surgical intervention in up to four metastatic lesions, but some surgeons contend that safe, effective radiosurgery can be performed for patients with up to ten metastases [35].




Fig. 13.3
Images of a patient with newly diagnosed lung cancer and solitary posterior fossa metastatic lesion. (a, b) Preoperative MRIs demonstrate (a) large enhancing mass on axial T1-weighted gadolinium-enhanced image; (b) coronal section of the same sequence both demonstrate significant mass effect and fourth ventricular compression. (c, d) Preoperative (c) T2-weighted and (d) FLAIR images demonstrate peritumoral edema. (e–h) Immediate postoperative (e) axial T1-weighted, gadolinium-enhanced, (f) coronal and (g) axial T2-weighted, and (h) FLAIR images demonstrate resection of the lesion, resolution of mass effect, and improving peritumoral edema

Fig. 13.4
Images of a patient with an asymptomatic ovarian cancer metastatic lesion to the left cerebellopontine angle . (a, d) Preoperative axial (a) and coronal (b) T1-weighted, gadolinium-enhanced MRIs demonstrating moderately sized lesion, with mild edema visible on axial (c) FLAIR and (d) T2-weighted images. Radiosurgery was performed to 1800 cGy to the 90% isodose line using dynamic conformal arc linear accelerator therapy (g). (e, f) Three-month posttreatment axial (e) and coronal (f) MRIs demonstrate significant decrease in size of the lesion. (h–i) Two-year follow-up axial (h) and coronal (i) MRIs demonstrate continued tumor control

Fig. 13.5
Images of a patient with metastatic colon cancer with controlled systemic disease and no neurological complaints or symptoms. Preoperative (a) axial and (b) coronal T1-weighted, gadolinium-enhanced MRIs demonstrate one small cerebellar lesion and one high parietal lesion. (c, d) Radiosurgical treatment of both lesions was performed. (e, f) MRI 3 years later demonstrate no new lesions and only small enhancing scar tissue with no evidence of tumor growth over this time period
Tumors of the posterior fossa represent a unique challenge for the neurosurgeon. Normally a contraindication to surgical intervention, multiple metastases or single, relatively inaccessible metastases may produce debilitating neurological symptoms that warrant surgical intervention despite no increase in patient survival and a greater chance of postoperative morbidity [36]. These cases should be approached judiciously; however, the opportunity to offer recourse from debilitating neurological impairment for a patient who has months to live cannot be overstated. A neurosurgeon must weigh all aspects of a patient’s condition, as well as his or her own technical abilities, and exercise the appropriate clinical judgment.
For treatment of brain metastases, management algorithms seek to provide evidence-based prognostic indicators to inform treatment decisions. The recursive partitioning analysis (RPA) classification scale devised by Gaspar and associates within the Radiation Therapy Oncology Group is a well-known prognostication tool [37]. Notably, the RPA for brain metastasis is divided into three prognostic categories, incorporating Karnofsky performance scale (KPS) metrics as well as age and systemic disease state (Table 13.1). Class I patients appear to benefit the most from any therapeutic modalities, such as surgery, SRS, or WBRT, and tend to have KPS ≥70, controlled extracranial disease, and an age of <65 years. Most patients fall into Class II, with ambiguous benefit depending on the patient, disease, and therapeutic options available. Class III patients (KPS <70) do not consistently benefit from therapy, no matter the modality, and have a median survival of approximately 2 months [37]. Therefore, the decision to operate must be considered within the context of the systemic disease, and more advanced systemic disease often predicts short-term survival regardless of intracranial tumor burden [38, 39].
Table 13.1
Recursive partitioning analysis classification scale for brain metastasis
Class | Patient characteristics | Proportion of patients |
|---|---|---|
I | KPS ≥70 | 20% |
Controlled primary disease | ||
Age <65 years | ||
No evidence of extracranial metastases | ||
II | KPS ≥70 | 65% |
Uncontrolled primary disease | ||
Age ≥65 years | ||
Other extracranial metastases present | ||
III | KPS <70 | 15% |
Because of the short overall median survival times often seen in patients with brain metastasis (8–12 months), much of a neurosurgeon’s efforts may be palliative and short; however, with appropriate patient selection, instances of long-term survivors will continue to increase. For patients with high tumor burdens and multiple metastases, SRS or WBRT may provide palliation of symptoms in sync with the medical or neuro-oncological team approach. Aggressive cytoreductive surgeries are often contraindicated in the context of the broader health of these patients, especially if they are neurologically compromised and have short life expectancy at the time of presentation [40].
Treatment options for patients with SBM include conventional chemotherapeutics (i.e., cytotoxic or hormonal) targeted at the specific type of tumor. Surgical resection is typically reserved for solitary or rapidly enlarging tumors that are causing a high degree of morbidity and decreased quality of life, although total resection of these lesions is often precluded by involvement of critical neurovascular structures. Radiation therapy, or SRS, is often the treatment modality of choice in these patients.
Perioperative Care and Surgical Techniques for Metastasis to the Posterior Fossa
Prior to the work of Harvey Cushing , tumor resection of posterior fossa lesions was seldom attempted because of the high morbidity and mortality associated with the procedure. Through meticulous documentation and perioperative care, coupled with new cautery instrumentation, Cushing added the practice of posterior fossa surgery into the neurosurgical armamentarium [44]. Much of today’s surgical decision making is influenced by understanding the precise three-dimensional location of a tumor and the posterior fossa. The posterior fossa is a special surgical situation where space is limited, and vital structures such lower cranial nerves and the brainstem are in close proximity. Successful surgical resection of metastatic tumors in the posterior fossa requires careful study and examination of tumor volume, location, and neighboring structures. The primary surgical objective should be safe gross total resection (GTR) for any metastatic lesion without incurring new neurological deficits.
Preoperative Care
Preoperatively , patients with posterior fossa metastatic lesions are started on dexamethasone to control vasogenic edema and brain swelling. Intraoperatively, mannitol, 3% saline, and mild hyperventilation may be required to relax the cerebellum during the dural opening. Patients who present with features of hydrocephalus and ventricular obstruction may have received an EVD preoperatively. Alternatively, a prophylactic burr hole can be placed intraoperatively 7 cm superior and 3 cm lateral to the inion to aid in placement of a ventricular drain for intracranial fluid management throughout the case. Surgical neuronavigation can aid in this process as necessary.
Surgical Approaches to the Posterior Fossa
Many surgical corridors to posterior fossa lesions have been described [45–51]. For the purposes of this chapter, we describe approaches that can be applied to metastatic lesions of the vermis, cerebellar hemispheres, and often deeper anatomy including the cerebellar peduncles, depending on the use of stereotactic guidance as necessary to define acceptable boundaries of resection. Approaches to the midline vermis , as well as the cerebellar hemispheres, can follow standard practices of suboccipital craniotomy and exposure. Lateral lesions, such as those near the cerebellopontine angle or jugular foramen, as well as deeper or anteriorly distributed metastases are accessed via retrosigmoid or skull base approaches. Frameless stereotaxis guided by thin-sliced MRI for real-time image guidance is used to avoid damaging important structures during approach and to ensure efficient and thorough tumor resection.
Posterior fossa craniectomy or craniotomy may be appropriate for single or multiple metastatic lesions that are surgically resectable. A recent retrospective, multivariate analysis of 88 patients undergoing surgical removal of metastatic posterior fossa lesions highlights a potential difference in patient outcome resulting from choice of surgical approach. The authors report a lower incidence of postoperative complications (12.5%) in patients receiving a craniotomy rather than craniectomy (34.6% overall complication rate). However, the relatively small number of patients and single-institution analysis precludes any strong conclusions based on this work. Importantly, mortality was unaffected by surgical approach [52]. Another group arrived at a similar conclusion in the pediatric population [53].
Proper patient positioning for posterior fossa craniotomies is important to provide clear working space, microscope visibility, and maneuverability of surgical instrumentation. Historically, three major positions have been used for surgical access to the posterior fossa: a park bench (lateral oblique), prone (or modified prone), or sitting position. Although the latter provides excellent exposure, a clear operative field, and venous drainage, it is associated with a risk of air emboli and surgeon fatigue after operating with outstretched arms. This approach has largely been abandoned by the senior surgeon and will not be further discussed in this chapter. A 3/4 prone position or lateral oblique position is preferred by the senior author for lateral hemispheric cerebellar lesions; however, in cases in which intraoperative MRI (iMRI) is used, this position is difficult because of limitations on the size of the MR bore (especially in larger patients), and a straight prone position has been adopted as a compromise. iMRI is an excellent adjunct to compensate for “brain shift,” a common phenomenon in posterior fossa surgery where cerebrospinal fluid (CSF) drainage, retraction, lesion excision, and brain edema are frequent occurrences. iMRI can compensate for these factors and ensure that complete removal of the lesion has been achieved [54].
Midline Approach for Vermial or Medial Hemispheric Lesions
For midline lesions, the patients are positioned straight prone on the operating table. Special attention must be given so that the skull is securely placed in the head-holding fixation device. Military flexion (axial distraction and head flexion) is obtained by allowing at least a finger’s breadth spacing between chin and chest. The venous jugular drainage and endotracheal tube are checked for any kinking, and the surgeon and anesthesiologist together confirm that ventilation and venous outflow are intact. For prone cases, a rigid or the so-called “armored” endotracheal tube may help with airway patency throughout the case. The table is elevated 10–15° above horizontal; with the patient’s head in about 20° of military flexion, there is a clear line of sight for midline approaches including C1 and foramen magnum anatomy when working under the operating microscopes. The patient’s legs may be flexed and supported with pillows under the shins. All extremities are padded, and the patient is securely belted or taped to the bed to allow for table rotation during the case for more avenues of visualization into the posterior fossa.
For medial lesions of the vermis or medial cerebellar hemispheres, a linear, medial incision is made beginning 2 cm superior and extending 6 cm inferior to the inion. Dissection of the soft tissue should occur along the nuchal line, carefully dividing it in the midline. Skin hemostasis is obtained with Raney clips, and the incision is retracted to provide access to the skull base for bony opening. The periosteum is reflected off of the bone with a periosteal elevator, and bone wax is used to occlude bridging and epiploic veins. The craniotomy or craniectomy (preferred by the senior author) is then performed, with special attention to remove all bone up to the venous sinuses (Fig. 13.6a). Neuronavigation can be used in these instances to verify vascular anatomy, especially if placing burr holes for a craniotomy; however, most of the time, with careful drilling and dural visualization, injury to the sinuses can be avoided. Small amounts of Surgicel or other hemostatic agents can be placed on any small areas of venous bleeding and with gentle pressure usually are sufficient to obtain hemostasis.
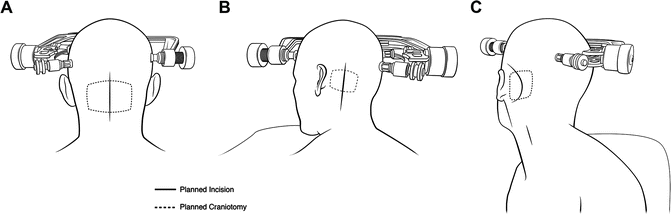

Fig. 13.6
Patient positioning and surgical approaches for lesions of the posterior fossa. Solid lines represent planned incision points. Dashed lines represent planned craniotomy/craniectomy areas. (a) Positioning , incision, and craniotomy/craniectomy for straight prone positioning for a midline or medial hemispheric lesion. Deeper midline lesions can be accessed through this approach, and the craniectomy may be extended inferiorly as far as the foramen magnum, often including C1 laminectomy for greater inferior exposure. Superior lesions of the cerebellar hemispheres may be accessible through this approach as well but necessitate tentorial retraction or transtentorial dissection. The incision in these cases may be moved progressively farther off of the midline to provide access to more anteriorly oriented lesions. (b) Park bench positioning for a lateralized lesion of the cerebellar hemisphere. Incision and craniotomy/craniectomy are made in relation to the mastoid notch but should be modified depending on the exact location of the lesion. (c) Modified supine positioning is useful for retrosigmoid approaches to far lateralized or anterior lesions of the cerebellum and/or brainstem . Incision and craniectomy are made in relation to the mastoid notch. A linear or curved (black line) incision can be made, depending on surgeon preference. Craniotomy/craniectomy can follow a similar path, using transverse and sigmoid sinuses as boundaries when possible. Importantly, the approach can be altered slightly depending on the anterolateral positioning of the lesion for maximal tumor exposure
The exposed dura is carefully examined for venous lakes and divided in a “Y”-shaped incision as possible to avoid these areas. The depth of the tumor will dictate its visibility at this stage, but careful surgical planning and extensive knowledge of posterior fossa anatomy often precludes excessive use of stereotactic navigation in many cases. For a deeper, midline lesions affecting the fourth ventricle, the inferior vermis may be divided at the midline, permitting resection of the tumor . The major concern with this approach is the risk of cerebellar mutism. Alternatively, a telovelar approach can be adopted to avoid splitting the vermis during resection of a fourth ventricular lesion. For deeper hemispheric lesions, the cerebellar cortex should be divided with a small corticectomy parallel to the cerebellar folia, and the tumor may then be visualized and resected. Neuronavigational tools may be useful here to define acceptable boundaries of resection, and intraoperative ultrasound can be used to localize tumors that are not easily visible at the surface. In select cases, iMRI can confirm adequate removal of tumor.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








