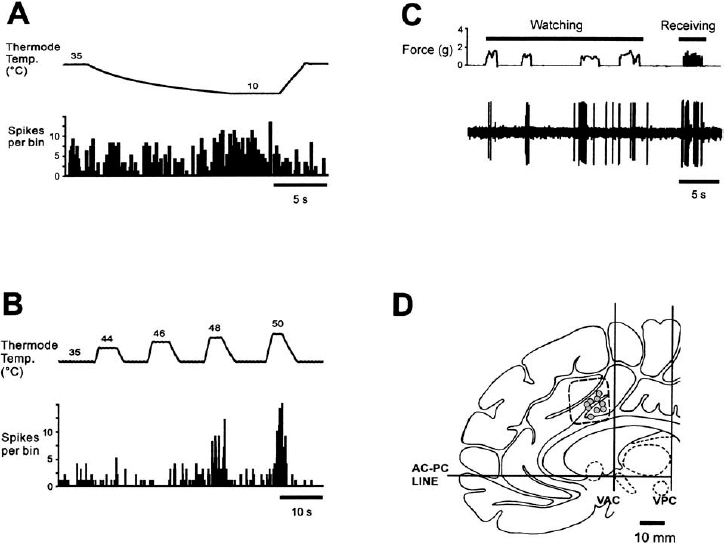
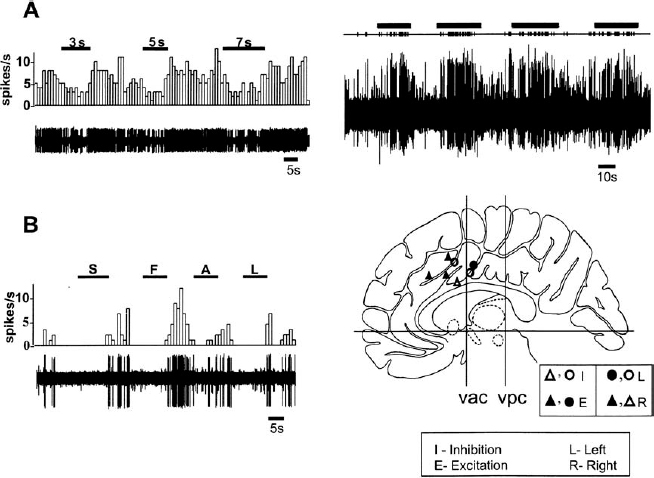
10 The following chapter details the electrophysiological findings in two disparate regions of the human brain: the cingulate cortex and the anterior thalamus. The clinical rationale for investigations in these two regions—the treatment of obsessive-compulsive disorder (OCD) and epilepsy, respectively—falls outside the traditional province of ablation and electrical stimulation for the movement disorders. For this reason, these two anatomically and electrophysiologically distinct regions will be considered together in this chapter. The cingulate gyrus lies deep on the medial aspect of each hemisphere, immediately dorsal to the corpus callosum. Contained within each cingulate gyrus is the white matter structure known as the cingulum, which is the principal association bundle of the medial aspect of each hemisphere. The cingulum connects the frontal and parietal lobes with the parahippocampal gyrus and with adjacent regions of the temporal neocortex.1 The cingulate cortex is divided into an anterior and posterior region, which are further subdivided into several cytoarchitectonic subdivisions: the anterior cingulate cortex (ACC) includes Brodmann’s areas 24, 25, and 32, and the posterior cingulate cortex (PCC) is subdivided into Brodmann’s areas 23, 29, 30, and 31.2,3 Horseradish peroxidase labeling studies in monkeys have revealed that area 24 is connected to the intralaminar, mediodorsal, and ventral anterior thalamic nuclei, to the amygdala, and to the nucleus accumbens septi. Area 23 is connected to the medial temporal and orbitofrontal cortices, the associative temporal cortex, and the medial pulvinar. Furthermore, areas 24 and 23 have been shown to be interconnected, and each to have connections with the caudate nucleus, claustrum, lateral frontal and posterior parietal (area 7) cortices, and “limbic” thalamic nuclei (AM, AV, and LD).2 In 1937, Papez4 hypothesized that emotional stimuli passed from the septum via the cingulum (within the cingulate gyrus), to the hippocampus, then via the fornix to the hypothalamus, then back to the anterior thalamic nuclei, and finally back to the cingulate gyrus. Subsequent stimulation5 or lesioning6,7 of the anterior cingulum in monkeys yielded autonomic responses associated with emotion or a decrease in fear and aggression, respectively. These findings led to the selection by Cairns8 of the anterior cingulum as a target for psychosurgery. Reciprocal pathways linking limbic structures such as the cingulate gyrus, hypothalamus, hippocampus, and amygdala, with widely distributed brainstem, striatal, paralimbic, and neocortical areas, have been defined in monkeys.9 Evidence of reciprocal connections in humans between the limbic system and the neocortex has been provided by positron emission tomography (PET) studies in the setting of depression and normal sadness.10 Mayberg and colleagues11 used PET to measure the time course of changes in brain glucose metabolism in unipolar depressed patients treated with the selective serotonin reuptake inhibitor fluoxetine. Time-specific and response-specific effects were examined at 1 and 6 weeks of treatment. Clinical improvement was uniquely associated with limbic and striatal decreases (subgenual cingulate, hippocampus, insula, and pallidum) and brainstem and dorsal cortical increases (prefrontal, parietal, anterior, and posterior cingulate). Failed response was associated with a persistent 1-week pattern and absence of either subgenual cingulate or prefrontal changes. The authors concluded that chronic treatment and clinical response to fluoxetine was associated with a reciprocal pattern of subcortical and limbic decreases and cortical increases and that failure to induce these changes may underlie treatment failure. The relief of intractable pain resulting from lesioning the ACC supports the role of the ACC in pain perception.12 Human imaging studies have demonstrated that painful stimuli cause activation of the ACC.13–19 Furthermore, stimulation of the sensory thalamus (Vc) in chronic pain patients by deep brain stimulation results in activation of the ACC.20 Bilateral cingulotomies have been used in the treatment of medically refractory chronic depression,21 OCD,22,23 pain,24,25 and generalized epilepsy.26 Additionally, Levin and Duchowny reported a case of a young girl with both medically refractory epilepsy and severe OCD.27 A scalp EEG and neuropsychological test scores suggested right frontal lobe dysfunction. The intractability of her seizures and her progressive intellectual and psychosocial deterioration prompted an evaluation for surgery. Intracranial EEG recording demonstrated a focal seizure origin in the right anterior cingulate gyrus. Cingulotomy resulted in postoperative seizure freedom and in a significant improvement in the patient’s OCD symptoms. In 1936, Egas Moniz published a report on the use of frontal leucotomy for psychiatric disease. The initial enthusiasm for this treatment of intractable psychiatric disorders and chronic pain was tempered by reports of undesirable side effects. This led neurosurgeons to search for modifications in the leucotomy technique that would increase safety without reducing efficacy. As a result of these clinical investigations, the original radical frontal leucotomy was replaced by small, stereotactically placed lesions in the limbic system. Ballantine and colleagues22 reported the results of stereotactic cingulotomy for the treatment of 198 “psychiatrically disabled” patients, evaluated prospectively for a mean follow-up of 8.6 years.22 Patients with major affective disorders and anxiety disorders fared the best, with a return to normal functioning in the majority. Although patients with OCD, schizophrenia, and personality disorders improved less overall, the relatively low mortality and morbidity of the more limited procedure, combined with a reduction in violent behavior, a possible reduction of suicidal risk, and a lessening of the intractable suffering of chronic psychiatric illness, suggested to the authors that cingulotomy could be an effective, safe treatment for patients with affective disorders that are unresponsive to all other forms of therapy. Cingulotomy remains in use today for the treatment of refractory OCD.23 In 1962, Foltz and White12 reported the relief of chronic pain as a consequence of bilateral stereotactic anterior cingulotomies. Faillace et al28 and colleagues found a 50% reduction in chronic pain levels in cancer patients, in response to stereotactic cingulotomies. More recently, Hassenbusch et al25 described the use of MR-guided stereotaxy for the placement of cingulotomies, as a means of replacing ventriculography in this procedure. Lenz and colleagues29 have reported that laser-induced painful cutaneous stimulation resulted in cerebral potentials (LEPs) recorded by subdural electrodes placed over the medial wall of the cerebral hemisphere. These LEPs were largest over the anterior cingulate and superior frontal gyri contralateral to the side of stimulation, providing evidence of significant direct nociceptive input to the human anterior cingulate gyrus (Brodmann’s area 24). Evidence of an important role for the ACC in attention, emotional self-control, focused problem solving, error recognition, and adaptive response to changing conditions has accumulated through single-neuron recording,30 electrical stimulation, EEG, PET, functional MR (fMR) imaging,31,32 and lesion studies.33 Reciprocal connections between the ACC and the lateral prefrontal cortex support a role for the ACC in cognition, as evidenced by the demonstration of functional connectivity between these two regions during the performance of cognitive tasks.34 Davis et al30 have provided direct evidence of an influence of a cognitive state on the spontaneous neuronal activity of neurons in the human ACC (Fig. 10–1). These investigators identified ACC neurons that increased activity in response to attention-demanding tasks, as well as other neurons that were inhibited by attention-demanding tasks. In humans fMRI during task-switching versions of the Stroop test has provided evidence of the role of the ACC in the monitoring of actions or performance in situations requiring adjustments in cognitive control.35 Other studies using event-related fMRI have provided evidence of the role of the ACC in detecting processes that conflict during task performance and signaling the extent to which attentional control is required.36,37 FIGURE 10–1 Inhibitory responses of a neuron in the human anterior cingulate cortex (ACC), during two different attention-demanding tasks (upper and lower left panels). The patient was instructed to perform mental arithmetic calculations (counting backward by 3, 5, or 7(A). and (B). to silently generate words beginning with the letter S, F, A, or L. Task periods are indicated by (—). The upper right panel shows the excitatory effect of increased attentional demand in a single ACC neuron, during the performance of mental arithmetic calculations (silent backward count). Each tick in the upper trace indicates the occurrence of an action poteninitial from a single neuron, extracted from the multiunit recording shown in the trace below. The lower right panel depicts the location of the ACC neurons that demonstrated modulation of activity during the performance of attention-demanding tasks. ▴ and Δ, right ACC; ● and O, left ACC; ▴ and ●, excitation; Δ and O, inhibition. (With permission from Davis KD, Hutchison WD, Lozano AM, Tasker RR, Dostrovsky JO. Human anterior cingulate cortex neurons modulated by attention-demanding tasks. J Neurophysiol. 2000; 83:3575–3577.) The patient is brought to the radiology suite on the morning of surgery, where a stereotactic frame is assembled and affixed to the patient’s head. MR images are then obtained. We use T2-weighted coronal images for target localization. The x, y, and z coordinates of the patient’s AC, PC, and midcommissural point (MCP) are not required for target localization, because the initial targets within each cingulate gyrus are selected directly from the coronal MR images. We choose an initial anatomical target situated 20 to 40 mm posterior to the anterior-most portion of the frontal horn of the lateral ventricles, near the ventral aspect of each cingulate gyrus, and 7 mm lateral to the midline. The MRI-based coordinates are then used to determine the coordinates of the initial target. The coordinates of the final target are determined by subsequent microelectrode recording data. Following the MR image, the patient is taken to the operating room and positioned on the operating room table. Using local anesthetic alone, two entry holes are made in the skin at the level of the coronal suture and 2 cm lateral to the midline, using a 4 mm punch. Bilateral twist-drill holes are made, and the underlying dura and pia are coagulated and pierced. Once the target coordinates have been selected based on the MR image, microelectrode recording is used to confirm the dorsal and ventral boundaries of the cingulate gyrus and to avoid surrounding structures (supplementary motor area and lateral ventricles). The stereotactic frame and arc are set to the x, y, and z coordinates of the target chosen on the MRI. A cannula is attached to the arc and lowered into the brain at an acute angle with the horizontal plane. A guide tube containing one or two microelectrodes is carefully inserted into the cannula and secured to the stereotactic arc. This procedure has been described in greater detail else-where.38 Continuous, extracellular recordings begin 10 to 15 mm above target and continue until 5 to 10 mm below target. Single- and multiunit neuronal discharges are amplified, filtered, displayed on an oscilloscope, and fed to an audio monitor. Based on qualitative audio monitoring, the discharge frequency and firing patterns of neurons (both spontaneous and evoked), as well as the relative size and shape of the action potentials, are all recorded. The electrode trajectory generally passes through the dorsal aspect of the cingulate gyrus, into the white matter of the cingulum, then into the ventral aspect of the anterior cingulate gyrus. Entry into the cingulum is marked by a decrease in background activity, reflecting the lack of somatodendritic activity in the white matter of the cingulum. Cells with receptive fields that respond to pain and attention-demanding tasks can be found within the cingulate gyrus.30,39 Neural activity again decreases as the electrode enters into the underlying white matter of the corpus callosum. The presence of cells that respond to active and/or passive movements suggests that the electrode is located within the supplementary motor area or primary motor cortex. High-frequency stimulation (100 μA; 1-sec train, 300 Hz, 150 μsec pulse width) is performed through the same electrode, but it has not yielded any consistent responses to date. Typically, only one electrode track is made per side. Although much information about the function of the human cingulate region has been obtained from functional imaging studies, information about the activity of individual neurons in this region requires microelectrode recording. During cingulotomies for either chronic depression or OCD, Hutchison and colleagues39 identified single neurons in the human ACC that responded selectively to painful thermal and mechanical stimuli, supporting a role for the ACC in pain perception. Eleven out of 63 neurons tested responded to contralateral painful thermal and/or mechanical stimulation, with 9 neurons exhibiting an increase in firing rate and 2 exhibiting a decrease. Neurons responded to two or three of the painful modalities tested (heat, pinprick, cold), and six neurons responded to ipsilateral as well as contralateral stimulation (Fig. 10–2). Additionally, three neurons seemed also to respond to the anticipation or observation of potentially painful stimuli. These authors noted that electrical stimulation of the ACC at various parameters did not result in painful sensations at sites that had been associated with neuronal responses to painful stimulation. In attempting to explain this observation, Hutchison et al raised the possibility that pain perception requires the simultaneous activation of other cortical regions in addition to the ACC, or, alternatively, that pain perception requires bilateral activation of the ACC. In another study by the same group, MER techniques were used to examine the effects of attention-demanding tasks on cingulate neuron activity.30 These investigators found that the activity of 7 out of 36 neurons tested (19%) was modulated by the performance of cognitive tasks but was unchanged by painful stimuli (Fig. 10–1). The cognitive tasks included mental arithmetic, the Stroop test (patients instructed to name the ink color of a word that is at odds with the actual word, e.g., yellow written in blue ink), and word generation (patients instructed to name all nouns belonging to a particular category, e.g., fruit, or all words beginning with a particular letter). The location of these attention-responding neurons overlapped with the region previously noted on fMRI to respond to similar tasks31 but was slightly anterior to the region noted on fMRI31 and with microelectrode recording39 to respond to painful stimuli. Thalamic stimulation has been used to treat patients with medically refractory multifocal and generalized epilepsy. Intrathalamic targets for the treatment of epilepsy have included the centromedian nucleus40–42 and the anterior thalamic nuclear group.43,44 At our institution, a clinical investigation of the efficacy of stimulation of the anterior thalamic nuclear group is currently under way for the treatment of medically refractory multifocal and generalized epilepsy. It should be emphasized that the efficacy of this procedure is still under investigation.
Microelectrode Recordings in the Cingulate Gyrus and the Anterior Thalamus
AVIVA ABOSCH, MOJGAN HODAIE, KAREN D. DAVIS, ANDRES M. LOZANO, AND JONATHAN O. DOSTROVSKY
The Cingulate Gyrus
Anatomy and Connections
Emotion and the Limbic System
Neural Circuitry of Pain and Cognition
Clinical Significance
OCD
Pain
Attention and Monitoring Behavior
Operative Technique for Cingulate Recording
Physiological Target Localization
Microelectrode Recording and Stimulation
Human Data
Thalamic Stimulation for Epilepsy
Neupsy Key
Fastest Neupsy Insight Engine