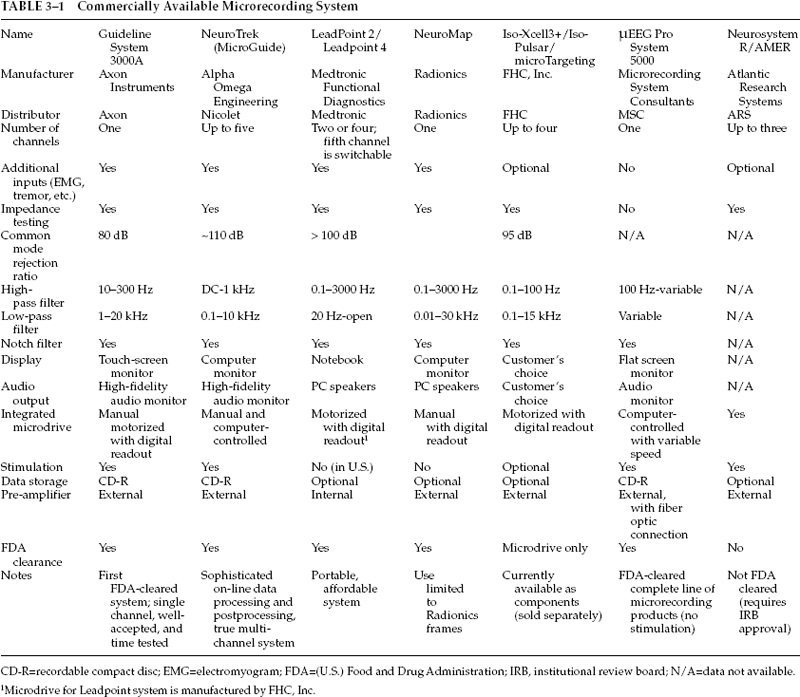
3 Microelectrode recording is an integral part of many surgical procedures for movement disorders. Technological advances and scientific discoveries in neurophysiology have resulted in wider availability of microrecording equipment and subsequent commercial development of multiple user-friendly systems for intraoperative microrecording. This chapter gives an overview of equipment needed for intraoperative microelectrode recording, describes its essential components, and compares several commercially available systems. To gain real-time information on electrophysiological properties of the deep cerebral structures, a system for microrecording must include the following components: (1) microelectrode or semimicroelectrode, (2) amplifier with appropriate filters, (3) oscilloscope or video monitor, (4) audio monitor, and microdrive for the electrode advancement. (5) In addition to this, there should be (6) a system for storage and archiving the recording data, (7) a computer for analysis of data, (8) a means to measure tissue and electrode impedance, and (9) a device for intraoperative microstimulation. In the past, clinicians and researchers had to create their own microrecording systems from separately purchased components. This practice resulted in a variety of setups, and many clinical centers are still using these “homemade” systems. Most of the commercially available microrecording devices (Table 3–1) are based on such systems, but smooth integration of functional components makes the recording procedure easier and eliminates some of the potential setup-related problems. Just as stereotactic surgeons should be familiar with principles of stereotaxis and details of the particular stereotactic frame they are using, it is also essential to understand the principles of microrecording and certain technical details of the recording equipment to avoid major instrumentation-related malfunctions and to perform on-site troubleshooting if such malfunction occurs. Conventional microelectrodes for intracerebral extracellular recording are designed to sense and transmit electrical activity of a neuron located in close proximity to the electrode tip. True microelectrodes have a tip diameter of ~5 μm, and those with tip diameter of 25 μm or more are considered “semimicroelectrodes.”4 Ideal electrodes9 should yield stable recordings with a high signal-to-noise ratio, be selective for activity from a single neuron, sample from all types of neurons, and be strong enough to withstand the rigors of in vivo use. Because some of the commonly used metals are either toxic for a human brain or fragile and unstable, the choice of the material for electrode manufacturing is rather limited. For example, silver, silver chloride, copper, and iron electrodes are known to be toxic, whereas gold, platinum, stainless steel, tungsten, and titanium electrodes are not. Because size and electrical properties of the microelectrode are critical for its performance, tungsten electrodes with high tensile strength that remain rigid as thin wire are currently used in most settings. Platinum has the advantage of being a catalyst for the electrolysis of water, thereby allowing dissipation of excess negative charge by forming hydrogen and hydroxyl ions instead of eroding metal from the tip,7 but pure platinum and even platinum-iridium alloy (the iridium is added for strength) are not as strong as tungsten. Therefore, tungsten microelectrodes are frequently plated with platinum, or gold and platinum, to achieve the desired electrical properties (impedance and ability to withstand repeated stimulation currents). Since the original description of tungsten wire microelectrodes in 19576 and of a simplified technique for manufacturing the glass-insulated platinum-iridium microelectrodes in 1960,10 these two electrode types have become widely accepted among clinicians.2,7,8,11,12 An interested researcher may find a detailed description of the microelectrode preparation technique in the literature7; those with less time and technical skill may consider purchasing ready-to-use electrodes from various manufacturers. A variety of microTargeting electrodes are available from FHC Inc. (Bowdoinham, ME). This manufacturer offers a choice of front-and back-loaded microelectrodes made of tungsten with epoxylite isolation, stainless steel with epoxylite isolation, and platinum-iridium alloy with glass insulation with different customizable length, tip exposure, and impedance. There are also shielded, bipolar, and “differential” electrodes. All these products are cleared by the U.S. Food and Drug Administration (FDA) for clinical use. The electrodes may be ordered with or without a sterilizable electrode tray that offers additional protection for fragile microelectrodes. Similarly, Atlantic Research Systems (Atlanta, GA) offers glass-insulated platinum-iridium electrodes of various lengths and impedances. Microrecording System Consultants (Pasadena, CA) also offers μElectrodes with a choice of different tip materials (tungsten, elgiloy, iridium, or platinum/iridium) and insulations (Kapton, epoxylite, or their combination). Impedance of microelectrodes determines recording sensitivity and also correlates with the amount of noise during recording. At a given frequency, the electrode impedance is inversely related to the surface area of the electrode/fluid interface. Therefore, to maintain high impedance, the exposed (free of isolation) surface of the electrode tip should be small. For the cone-shaped tip, a platinumiridium electrode will have an impedance of 5 megaOhm (MOhm) at 1 kHz if the cone height is 14 μm and the cone base is 5 μm.9 Covering the electrode tip with platinum black may reduce impedance significantly; this property is useful in cases where lower impedance is desired. Most commonly used electrodes have impedances of 1 to 1.2 MOhm at 1 kHz. This value is preferred because lower impedance will not allow recording from a single cell, whereas higher impedance electrodes will generate excessive noise when multiple electromagnetic devices (operating table, overhead lights, patient monitors, computers, sequential compression boots, bipolar coagulators, suction pumps, etc.) are present.2 Because the electrode impedance affects the clarity of microrecording, most commercially available microrecording systems include an option of on-line impedance measurement; but the process of impedance measurement may change electrode impedance and affect the quality of microrecording. With this in mind, some manufacturers recommend against direct impedance measurement and instead estimate impedance by examining the electrode tip under a microscope and measuring the area of the exposed cone. Activity of a neuron (single unit) captured by a microelectrode has to be amplified and cleared of inevitable electrical noise using a system of amplifiers and filters. The initial process of preamplification is usually done by a device positioned in close proximity with the stereotactic arc, or on the arc itself, to minimize the length of wire and cables and reduce environment-related noise. This preamplification is performed by a so-called operational amplifier (op amp),7 which is frequently used for biological recordings. All op amps require some input current to activate them; ones based on bipolar transistor inputs need much more current than those based on field effect transistors (FETs),13 making FET-based op amps more suitable for microrecording purposes.11 A preamplifier works by amplifying the difference between two inputs; this process is frequently based on a principle of “common mode rejection”: the noise consisting of large-amplitude and low-frequency sinusoid signals is common for both active (the microelectrode tip) and inactive (reference tubing) contacts and is eliminated by subtraction of one signal from the other. In the Guideline 3000A system (Axon Instruments, Inc., Union City, CA), a preamplifier is housed in a headstage that is usually placed close to the stereotactic frame.14 The headstage has appropriate cable connections and does not have to be sterile; in fact, a 6-foot-long cable allows one to place the headstage quite far from the sterile field. For the NeuroTrek system (Alpha Omega Engineering, Nazareth Illit, Israel), currently marketed in the United States as MicroGuide (Nicolet Biomedical, Madison, WI), the headstage is further miniaturized. It is sterilizable and meant to be taped to the frame in immediate proximity to the microdrive and the microelectrodes. The μEEG Pro System 5000 (Microrecording System Consultants) has an external differential preamplifier that digitizes the signal at the headstage, then transfers it directly to a computer via a fiberoptic connection, providing electrical isolation between the system and the patient. The Leadpoint system manufactured by Medtronic Functional Diagnostics (Shoreview, MN) does not have an external preamplifier. Its preamplifier is internal and is also electrically isolated. In addition to a simple signal amplification and common mode rejection noise subtraction, the microrecording system should eliminate that part of the raw signal that has little or no physiological value. Very high and low frequency signals of this type obscure the single-cell discharges that are characterized by a certain frequency range (usually 200 to 10,000 Hz). To accomplish this, the system “purifies” the signal using low-pass and high-pass filters that remove high-and low-frequency signals, respectively7 Although they produce some phase distortion, Butterworth-type filters are quite acceptable and are frequently used in “homemade” and commercially available systems. In the electrically noisy operating room environment, interference from the alternating current (AC) lines may remain a challenge despite the use of the high-pass filters. This particular noise may be eliminated by using a dedicated “notch filter” that specifically filters out the offending 50 or 60 Hz (depending on the country) frequency signal. This notch filter, and sometimes a high-pass filter as well, may need to be turned off when recording low-frequency signals, such as optic tract potentials. During this stage of a surgical procedure, additional effort should be made to decrease AC noise by turning off all nonvital electrical equipment, such as blood pressure monitors, overhead lights, and operating table motors. An experienced neurophysiologist may identify different parts of the central nervous system just by listening to the electrical activity. Most subcortical regions, particularly the thalamus and the basal ganglia, have specific and somewhat unique electrical discharge patterns, or signatures, that may be recognized by the trained ear of an experienced clinician or researcher. However, this combination of experience and intuition may not suffice for making important surgical decisions, therefore necessitating the introduction of technical and scientific instruments to define this discharge pattern. The signal after amplification and filtering undergoes immediate on-line analysis, allowing one to identify single cells and characterize them. The “spike-discriminator” software sorts the discharges by their characteristics; in essence, this is a circuit that looks at the amplified and filtered signal from the electrode and generates a logic pulse whenever a transient in the signal satisfies a predetermined set of criteria.13 These criteria usually include amplitude and time course of the discharge. In a simpler model, the circuit identifies all discharges, with amplitude intermediate to the operator-set lower and higher voltage levels. When the spike crosses the lower level and then recrosses it without reaching the upper level, a logic pulse is triggered. This method is sensitive but inevitably results in a delay between the spike and its appearance on the screen of the oscilloscope. In more sophisticated spike discriminators, the spikes are distinguished based on both amplitude and time course, therefore allowing one to separate spikes with similar amplitude but different time parameters. In addition to crossing a voltage threshold, the spike should fit into a certain voltage or time window to trigger the output pulse.13 This model, originally developed by Bak and Schmidt,1 is now widely used in various systems. The spikes that are identified by this discrimination technique may be played out using a delay-line feed into an oscilloscope; the triggering pulse allows for displaying only discriminated spikes. If one discriminated spike is saved on the screen, other spikes may be superimposed over it and compared. Specially designed software helps to sort spikes and to calculate their frequency and other parameters. Other processing algorithms are used to create a histogram of spikes that is helpful in evaluating the pureness of the recording and the number of recorded cells; to display a digital raster of discharges, which may help in assessing a discharge pattern, the length of pauses, and the bursting episodes; to calculate and record the interspike interval that quantifies the discharge patterns; and to provide a computational simplification of recorded signal using the fast Fourier transform (FFT) analysis.5 This last function allows the computer to decompose or separate a waveform into sinusoids of different frequency that sum to the original waveform. FFT is an algorithm for discrete Fourier transform that was developed in 1965 by Cooley and Tukey3 to reduce the number of computations. With current computer technology, this analysis may be performed almost immediately in an on-line fashion. FFT distinguishes the different frequency sinusoids and their respective amplitudes, allowing real-time display of the signal characteristics. In addition to the commonly used on-line analysis of the microrecording data, many centers perform “off-line” analysis, including digital conversion of the signal, analysis of firing patterns, mapping, and interspike intervals.4 To facilitate this, most microrecording systems have the capacity to store and archive intraoperative information on hard drive or recordable compact disc (CD-R) [standard configuration (Axon, Alpha Omega, MRC) or as an option (Medtronic, Radionics)]. To make this processing easier, various events may be marked with textual or audio messages attached to the data. The format of recorded information varies from system to system (.abf files in Axon, .map files in Alpha Omega, etc.), but in most cases this information may be read by various data analysis programs. Although a standard oscilloscope may be sufficient for visual representation of neuronal activity, a digitally processed signal is better displayed on dedicated computer screens and monitors. Most commercially available systems include such monitors. In addition to real-time graphic images of neuronal discharges, these monitors display a variety of useful information such as electrode position, microrecording signal characteristics, patient data, control buttons, and results of different analytic processes. Most commonly, there are various histograms, raster displays, and graphs that are arranged in separate resizable windows. High-resolution multicolor monitors provide better graphical images but occupy significant space, requiring large racks and trolleys, whereas laptop computer screens are more compact and easier to store and transport at the expense of lower resolution and image quality. Some systems (e.g., MicroGuide by Alpha Omega) may save an entire screen image as a bitmap file along with the recording data. This feature may be useful for the event recall during postprocessing. Touch-screen displays (such as in Guideline 3000A by Axon Instruments) facilitate intraoperative equipment control, eliminating the need for multiple buttons, handles, and dials. The sterility of the operator, however, remains problematic. Some systems, therefore, provide additional control pads that serve as primitive keyboards or computer mice/trackballs and may be handled through a sterile sleeve or, if they are sterilizable, may be passed onto the sterile field itself. In regard to the audio monitor, this part of the microrecording system may be essential for many clinicians and neurophysiologists who are trained for aural processing of the recorded signals. High-fidelity speakers may be set up for real-time play of the raw neuronal signal (or so-called neuronal noise in case of semimicroelectrode recording) or, at the operator’s choice, for a particular type of filtered information, such as spikes of certain characteristics. The latter modality is especially useful in cases of rhythmic cellular activity such as tremor or movement-associated discharges. If one wants to avoid additional noise in the operating room or to achieve higher clarity of the signal, the single or multiple headphones may be used instead of the external speakers. An integral part of intraoperative physiological localization is the electrical stimulation of deep cerebral structures. Because it may be performed in tandem with microrecording, some microrecording systems provide the option of using microelectrodes for the stimulation. This procedure is considered microstimulation if it is done through the tip of the microelectrode, or macrostimulation if the stimuli are delivered using the external cannula or some different, larger electrode (usually the one used for subsequent radio frequency lesioning). There are several characteristics that define micro-and macrostimulation. Stimulus waveform and polarity are usually preset for each system, whereas amplitude of the stimulation, pulse duration, frequency, and the train duration may be adjusted within a certain range. The Guideline 3000A (Axon Instruments) system, for example, has a choice of four amplitude ranges (from 0–10 μA to 0–100 μA) that limit manual adjustment of the stimulus current using a rotating knob at the handheld remote control. Frequency of stimulation (1–300 Hz), pulse duration (0.05–1 msec), and train duration (manual or 0.5–20 sec for symmetrical biphasic current pulses) may be set using a control panel of the stimulator. The MicroGuide system (Alpha Omega) has similar capabilities in adjustment of pulse width, frequency, and duration of stimulation; it also gives a choice between biphasic and monophasic stimulation and lets the operator adjust the stimulation current by pressing up and down buttons instead of using the rotating knob. The ranges for the stimulation current choice are somewhat higher (0–100 μA, 0–1 mA, 0–10 mA), thus providing the ability to perform “macrostimulation” during the recording session. One would normally prefer to avoid using high-stimulation currents while using microelectrodes because this will definitely affect the electrode impedance and impair further recording with this electrode; it also causes extremely high voltage of stimulation that may potentially endanger surrounding neural tissues. Therefore, higher current stimulation is usually done using outer cannulas (or dedicated macroelectrodes) after microrecording and microstimulation are completed. If the microrecording system does not support microstimulation (e.g., Leadpoint by Medtronic and μEEG Pro by MRC), a stand-alone stimulator may be used (such as Pulsar by FHC), but these stimulators are frequently not cleared by the FDA and may require specific approval by an institutional review board (IRB) for human application. To safely navigate the microelectrode inside the human brain in vivo, one needs to have a reliable and precise means to move the electrode in and out along a predetermined trajectory. It is also important to know the depth of the electrode tip to correlate its position with certain anatomical regions and structures. The electrode must be very steady in the lateral and anteroposterior planes to avoid an undesired leucotomy effect. All this may be accomplished by using a dedicated mechanism for electrode advancement and withdrawal—a microdrive—that is attached directly to the stereotactic frame. A survey of neurosurgical practice done in 1995 indicated that the majority of microdrives at that time were hydraulic, with only few centers using step-motor devices.4 One of the advantages of the hydraulic system is its electrical indifference; manual rotation of the wheel is translated into smooth advancement of the microelectrode without any electrical interference. It is possible to continue microrecording while the microelectrode is moving so that the movement may be halted as soon as the electrode reaches position in close proximity of a discharging neuron. The position of the electrode is usually read directly from the drive’s wheel or drum, or from a digital micrometer attached to the microdrive. Electronic digital micrometers provide readings with precision of 1 micron. Relatively inexpensive microdrives are available from several manufacturers, including FHC (models 50–12–8 and 50–12–9) and Stoelting (Wood Dale, Illinois) (model 51421) (Fig. 3–1), but neither of these two is FDA approved for use in humans. Motorized microdrives are usually based on a stepper motor principle. In addition to variable speed of the electrode advancement, they come with an adjustable step size, which allows the operator to move an electrode with high precision using a handheld remote. Although movement of the stepper motor is governed by computer software and electronic controls, the depth of the movement is usually measured by a dedicated linear potentiometer that provides absolute linear coordinates of the electrode position (Z axis). This principle is used on most commercial microdrives supplied with MER systems. The range of vertical movement varies from system to system. Referred to as the electrode travel distance, this range is set between 50 and 70 mm. There are two approaches to the horizontal and anteroposterior positioning of the microelectrode (X and Y axes). The first approach is to use a manually adjusted X-Y stage that accommodates one or two electrodes and has a freedom of movement between 5 and 10 mm in each direction. This movement is controlled by micrometer screws and must be completed prior to the insertion of the electrodes and guide tubes. The second approach is based on preset lateral and anteroposterior positions of multiple electrodes relative to the central (target) electrode. An example of such a configuration is the BenGun, developed by Benabid in Grenoble and included in the MicroGuide system (Alpha Omega). This device accommodates five electrodes, including a central electrode and four others positioned 2 mm anteriorly, posteriorly, laterally, and medially to the central electrode. This system allows simultaneous advancement of up to five electrodes parallel to each other. These two approaches may be combined if the BenGun device is attached to an X-Y stage with additional 10 mm movement in each direction. Obviously, the target coordinates may be adjusted by changing the position of the stereotactic arc, but using X-Y stages and BenGun-type devices eliminates the need to manipulate the frame that may create potential problems with sterility of the field and operator convenience. Key elements of the microdrive may be sterilized. It is extremely important, however, to strictly adhere to the manufacturers’ specifications to avoid damage to the fragile electronic components during the sterilization process.
Microelectrode Techniques: Equipment, Components, and Systems
KONSTANTIN V. SLAVIN AND JAMES HOLSAPPLE
Microrecording Systems
Microelectrodes
Signal Amplification and Filtering
Signal Processing
Video and Audio Monitors
Micro- and Macrostimulation
Microdrives
Neupsy Key
Fastest Neupsy Insight Engine