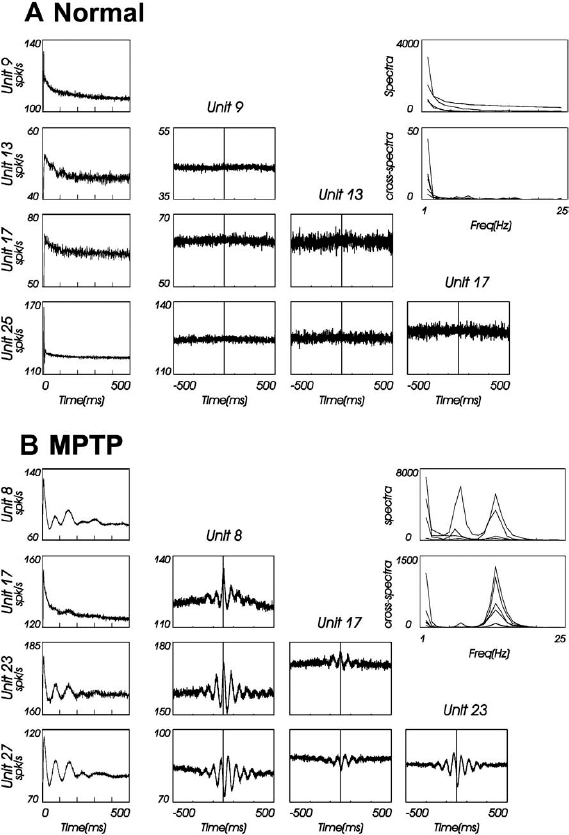
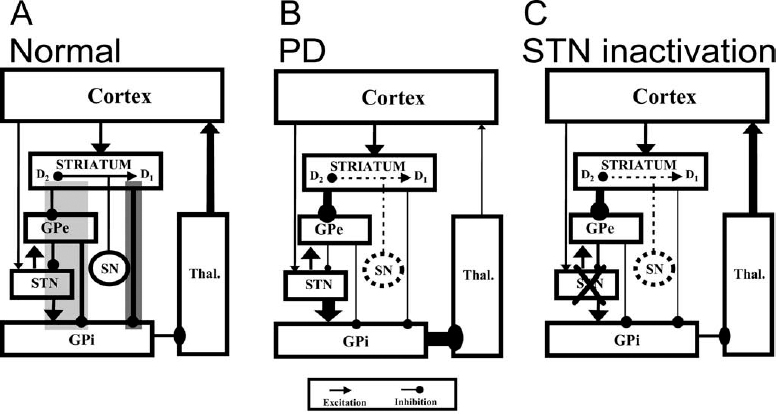
6 The development of the primate 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) model of parkinsonism in the early 1980s1,2 has led to great progress in our understanding of the pathophysiology of Parkinson’s disease (PD). In particular, application of the microelectrode recording (MER) technique to this model has uniquely contributed to our current understanding of the function and dysfunction of the basal ganglia (BG). Furthermore, MER in MPTP primates has enabled the systematic reevaluation of old surgical therapies for PD and for levodopa-induced dyskinesia (LID), such as pallidotomies and thalamotomies, and to the advent of novel ones, notably the introduction of the subthalamic nucleus (STN) as a preferred site for surgical treatment of PD. In this chapter we will outline the major electrophysiological findings gleaned from MER in the MPTP primate model of PD. Through these findings we will introduce various organizational and functional principles of the BG, as they have been formulated in current models. We will also compare these data to electrophysiological data gathered from human PD patients undergoing stereotactic surgery. The chapter will be organized as follows. In the next section, we will give a brief description of the MPTP primate model. We will then describe the effect of MPTP on tonic firing rates throughout the primate brain and how neurosurgical therapies aim to normalize them. We will also discuss the temporal aspects of neuronal discharge in MPTP primates and how these are affected by dopamine replacement therapy. Next, we will present spatial aspects of neuronal discharge in the corticobasal ganglia (CBG) circuit of these primates and their functional implications. This will require the introduction of the multiple MER (mMER) technique as an important experimental probe of functional connectivity in these circuits. We will review current computational models of BG function that incorporate essential findings from MER in humans and primates, then conclude with an outline of prospective areas of research for MER in MPTP primates. The relevance of MPTP to PD became clear in 1982 when this substance contaminated batches of a synthetic heroin analog that was subsequently self-administered by several drug addicts. These individuals proceeded to develop all the cardinal symptoms of PD, including bradykinesia, akinesia, and muscle rigidity. Several of them also exhibited some degree of resting tremor.3,4 This unfortunate incident marked the beginning of a new era in PD research, with the introduction of a promising new primate model for the disease.1,5–9 Monkeys treated with MPTP exhibit most of the cardinal symptoms of PD, including akinesia, bradykinesia, hypometric movements, cogwheel rigidity, flexed posture, and loss of facial expression.1,8,10–13 Electromyographical (EMG) activity becomes disorganized and displays co-contraction of antagonistic muscles.10,14,15 Although low-frequency (4–8 Hz) resting tremor is not readily replicated in this model,2,16–18 some species, notably the African green (vervet) and, to some extent, Rhesus monkeys, can develop a prominent postural/action tremor. These monkeys display episodes of tremor at frequencies in the 5 to 15 Hz range.17,19–24 MPTP crosses the blood-brain barrier and is converted into an active metabolite, which is then selectively taken up by the dopaminergic neurons of the midbrain. Inside these cells it causes oxidative stress, leading to cellular damage and death.25–27 Postmortem examination of the MPTP-treated monkey’s brain reveals that the primary insult is caused to the dopaminergic system and demonstrates that the extent of neuronal damage depends on the comprehensiveness of treatment. Tyrosine hydroxylase (TH) immunoreactivity reveals loss of TH-positive staining in the terminal fields of the dopamine neurons in the striatum, as well as in their cell bodies in the substantia nigra.28–30 The extent of neuronal damage and the severity of the resulting parkinsonian symptoms strongly depend on the protocol used to administer the MPTP. Several classes of protocols exist. The most common protocol is the systemic injection of MPTP. Depending on the total dosage and the number of injections used, these monkeys can develop a full spectrum of responses, ranging from severe parkinsonism24,31 to a mild clinical state dominated by frontal cognitive deficits but lacking motor signs.13,32–36 Chronic low-dose MPTP treatments have been suggested as a more reliable model for the slowly progressing human disease.33,37,38 In contrast with the human pathology39 acute MPTP treatment produces dopamine depletion that is equal to or more severe in the caudate nucleus than that in the putamen.25 In animals chronically treated with low doses of MPTP, there is a greater decrease of dopamine in the dorsolateral (putamenal) portions of the striatum. Frontal cognitive deficits and abnormal eye movements can be observed in these animals even before the development of motor deficits.13,32,40 This is in line with early cognitive deficits found in human MPTP41,42 and PD patients.43 Other methods include administration of MPTP by intracarotid injections44,45 or by striatal mini-pumps.46 These methods are very useful because they produce hemi-parkinsonism, enabling an easier control of the animals’ health and feeding. However, the confounding compensatory interaction between the two hemispheres and the bilateral (although not symmetric) nature of the human disease suggest that the systemic MPTP models may be closer to the human pathology. MPTP primates are used in all experimental aspects of PD research: anatomy,25,29,30 pharmacology,47–50 cranial metabolism,51,52 neural grafting,53,54 and neuroprotection.55,56 However, their prominent role has been in electrophysiological studies. MER in MPTP monkeys has enabled the systematic study of how neuronal discharge throughout the CBG circuit is altered in parkinsonism. This work has formulated our current understanding of the pathophysiology of PD and has enabled the systematic testing of surgical procedures aimed at alleviating parkinsonian symptoms.57–60 The MER findings from MPTP primates that have made the most impact on PD research pertain to changes in tonic firing rates throughout the CBG circuit. Several primate studies have shown that MPTP has a differential effect on firing rates in the internal and external segments of the globus pallidus (GPi and GPe, respectively). The tonic firing rate of GPi neurons increases from ~80 spikes/s to ~100 spikes/s (15–40% increases),17,61–65,65,66 while the rate of GPe neurons decreases from ~70 spikes/s to ~50 spikes/s (25–33% decreases).17,24,61,62,64,65 The tonic firing rate of STN neurons increases after MPTP from ~20 spikes/s to ~25 spikes/s (15–65% increases).17,22 The STN and pallidal neurons recorded in these studies are probably projection neurons that form the circuits originating in the cortex and traversing the BG. The pallidal projection neurons are GABAergic (γ-aminobutyric acid), and probably inhibitory, whereas the STN projection neurons are glutamatergic and excitatory. The changes in the spontaneous firing rates of striatal neurons following dopamine depletion are less clear than the above-mentioned changes in GP and STN. One study has claimed that the spontaneous firing rates of striatal neurons in MPTP primates decrease from 6 to 4 spikes/sec.67 However, it is not clear whether a distinction was made in this study between the phasically active neurons (PANs) and the tonically active neurons (TANs) of the striatum.68,69 The PANs are the GABAergic projection neurons of the striatum (also called the medium spiny neurons). These cells fire phasically in response to behavioral events70–73; however, their spontaneous firing rates are low (< 1 Hz). Even though they constitute ~95% of striatal neurons, they are extremely difficult to isolate for stable recording in the normal and the MPTP-treated monkey. Studies in MPTP cats, in contrast, have shown that the spontaneous firing rates of striatal PANs increase from 2 to 6 spike/sec.74 Similarly, dopamine inhibits 75% of the spontaneously active striatal cells in awake, unrestrained rats.75 Recent in vitro intracellular recordings revealed that membrane potentials of striatal projection neurons in rats with chronic 6–hydroxydopamine nigral lesions were more depolarized, resulting in an increased firing rate of these neurons.76 In summary, it is still unclear how the firing rates of striatal projection neurons in the primate respond to dopamine depletion. In contrast to striatal PANs, the response of primate striatal TANs to MPTP is well documented. These cells are large and easily isolated for recording. They are probably the cholinergic interneurons of the striatum and constitute ~2% of striatal neurons69,77,78 The basal firing rate of the TANs is not affected by MPTP treatment79,80; however, their firing patterns and synchronization are modified. We will return to these neurons later. FIGURE 6–1 The Albin-DeLong model of the corticobasal ganglia (CBG) circuit. (A). The CBG circuit is comprised of a feed-forward loop leading from the cortex to the BG, then back to the frontal cortex via the ventrolateral nuclei of the thalamus (Thal.). The striatum is linked to the internal segment of the globus pallidus (GPi) via the direct (dark highlight) and the indirect pathways (gray highlight). The indirect pathway traverses the external segment of the globus pallidus (GPe) and the subthalamic nucleus (STN). Dopamine innervation from the substantia nigra (SN) is mediated by the D1 and D2 receptors acting along the direct and indirect pathways, respectively. Arrowheads and circle heads mark excitatory and inhibitory links, respectively. (B). In Parkinson’s disease (PD), the loss of dopamine innervation weakens the direct and strengthens the indirect pathways, resulting in disinhibition of the GPi, which becomes overactive. This leads to excess inhibition of the thalamocortical pathway, leading to parkinsonian hypokinesia. (C). Inactivation of the STN reduces the excess excitation to GPi, which normalizes its inhibitory output, resulting in the amelioration of PD symptoms. These results are best understood in the framework of the Albin-DeLong model of the CBG circuitry, which is depicted in Figure 6–1A.81,82 The Albin-DeLong model ignores many of the inter- and intranuclei connections,83,84 but depicts the general feed-forward loop structure of this circuit. This model highlights two segregated striatal pathways that converge on the GPi. The first is the disynaptic inhibitory pathway from the cortex to the GPi via the striatum. This pathway is coined the direct pathway. The second pathway is the indirect pathway, which is a polysynaptic disinhibitory pathway from the cortex to the GPi via the striatum and the GPe. One branch of this pathway includes the STN. Finally, we depict the purely excitatory cortico-STN pathway, recently labeled the hyperdirect pathway,85 even though this pathway was neglected in the original formulation of the model.81,82 The cortico-STN input to the circuit is anatomically distinct from the direct and indirect striato-GPi pathways. Early histochemical and pharmacological studies suggested that the direct and indirect striatopallidal pathways are also segregated from each other. It was shown that two populations of striatal GABAergic projection neurons exist and that each is identified with one of the striatopallidal pathways. The projection neurons in the direct pathway contain substance P and dynorphin and express D1 DA receptor subtypes, whereas those in the indirect pathway contain enkephalin and express D2 DA receptor subtypes.86,87 Furthermore, it was suggested that nigral DA has a differential effect along the two striatopallidal pathways: it facilitates transmission along the direct pathway via the D1 receptors and inhibits transmission along the indirect pathway via the D2 receptors.81,82,86,88 Indeed, the MPTP-induced changes in firing rates described above provide strong support for this model, as they are readily explained by it (Fig. 6–1B). The decrease in DA levels (due to the MPTP insult) leads to an effective weakening of the direct pathway (less excitatory D1 modulation of striatopallidal neurons) and to a strengthening of the indirect one (less inhibitory D2 modulation of striatopallidal neurons). As a result, the GPe is excessively inhibited, resulting in a decrease of its basal firing rate. The STN is disinhibited, due to the decrease in the GABAergic output of the GPe, and thus increases its firing rate (Fig. 6–1B). Finally, the GPi is overexcited, due to the increased STN activity, and is disinhibited via the direct striatopallidal pathway, resulting in an increase of its basal discharge rate. The effective excitation of GPi presumably leads to excessive inhibition of the frontal cortex, which in turn leads to parkinsonian akinesia, bradykinesia, and rigidity. An important prediction followed the finding of abnormal tonic firing rates in the BG of MPTP monkeys: ablation or inactivation of GPi or STN should lead to the alleviation of parkinsonian symptoms. The logic was solid. Lesioning of the GPi would remove the excessive inhibitory drive to the frontal cortex (via the thalamus), hopefully resulting in the amelioration of PD symptoms. Similarly, destroying the STN would transect the indirect pathway and would, more importantly, remove the excessive excitatory drive to the GPi. This would normalize GPi output, thereby reducing the inhibition of frontal cortex (Fig. 6–1C). These predictions were in fact borne out. Pallidotomies, which were largely abandoned since the advent of levodopa therapy, were shown to be very successful at alleviating akinesia and rigidity of human patients.89–91 Injection of excitatory amino acid antagonists into the GPi of MPTP-treated monkeys were shown to reverse the motor symptoms of parkinsonism.92 STN lesions and inactivation (by injection of the GABA agonist muscimol) were shown by several groups to reverse parkinsonian symptoms in MPTP primates.93–97 The primate results set the stage for the introduction of subthalamotomies as treatment for PD patients98,99 and for the development of deep brain stimulation (DBS) of the STN as an alternative treatment for PD. DBS of the STN or of the GPi has been successful at alleviating parkinsonism both in PD patients100–102 and in MPTP primates.103 There are indications that GPi stimulation is best suited to treat LID and rigidity, whereas STN stimulation is best for the treatment of akinesia, rigidity, and tremor.100–102,104 Today DBS is preferred by neurosurgeons over ablative surgery because of its reversibility and parameter-tuning capabilities.57,59 What, then, is the effect of DBS on neuronal discharge? This question dates back to Ranck105 and Asanuma et al.106 They showed that when metal microelectrodes (exposed tip ≅ 5–15 μm; impedance ≅ 0.5–10 MΩ at 1 kHz) are used, the susceptibility of nerve fibers is much higher than that of the cell bodies, suggesting that microstimulation activates bypassing fibers. Nevertheless the classical interpretation of the effect of microstimulation on the motor cortices has been that the stimulation induces excitation of the cortex or corticospinal axonal pathways and thus evokes movement of different body parts. Because the effect of DBS in PD is paradoxically similar to the effect of lesions (e.g., neuronal inactivation), the question of the actual effect of DBS has been addressed recently both in the MPTP primate model and in human PD patients. In the MPTP primate, DBS in the GPi has been shown to bring down the mean GPi firing rates to within the normal range.63 Similar results have been described recently in rodent studies107 and in human PD patients.108 There are several possible mechanisms to explain these results: depolarization block of the stimulated neurons, stimulation of bypassing inhibitory pathways, and/or induction of GABA release from the terminals of the GPe projection neurons, thereby inhibiting the target GPi neurons. A major difference between human DBS and primate or human microstimulation is that the former uses macroelectrodes (with impedances in the ranges of a few kΩ). The current densities around the stimulating electrodes are very different in micro- and macrostimulation. Thus, in a recent human study, GPi stimulation (verified by MRI) was found to exacerbate akinesia, whereas GPe stimulation alleviated it. These results led to the conclusion that this stimulation presumably activated the neurons themselves, although no direct electrophysiological evidence of this activation was available.109 Application of DBS to the STN of MPTP primates has generated conflicting results. In an early study, DBS was found to differentially affect the mean discharge rates in the GPe and GPi for several hours after the DBS: it caused an increase in the former and a decrease in the latter.110 In a recent study the mean firing rates increased in both segments during DBS.111 DBS with macroelectrodes has been shown in rats in vitro to directly activate the membrane potential of the STN neurons.112 In summary, the precise neuronal mechanism of DBS (e.g., effects on neurons or fibers in the area of the electrode, effect of the stimulated area on remote structures, etc.) is still an open issue. Despite this model’s usefulness, it runs into several problems. Application of dopamine to striatum does indeed produce mixed responses among striatal neurons113,114; however, the prediction of D1-induced excitation versus D2-induced inhibition of striatal neurons has not been verified by direct electrophysiological measures. Furthermore, because these striatal projection neurons are quiescent or have very low basal firing rates, it is unclear how they would be able to drive the disinhibition of the GPi via the direct pathway, as described by the Albin-DeLong scenario of parkinsonism. In addition, as discussed above, it is not clear whether and how firing rates of striatal projection neurons change in response to MPTP treatment. Recent works have further challenged the basic tenets of the Albin-DeLong model. Several MER studies have failed to find a significant decrease in GPe firing rates in all the MPTP-treated monkeys examined,24,66,115 and other studies have failed to find a significant increase in GPi firing rates in all the MPTP monkeys.22,24,116 Similarly, biochemical and metabolic studies indicate the GPe activity does not change in parkinsonism.117 The model strongly predicts that the enhanced GPi inhibitory output in parkinsonism should reduce motor cortex firing rates, resulting in akinesia, bradykinesia, and rigidity. However, several works in dopamine-depleted primates have shown no change in spontaneous motor cortical firing rates.14,15,118,119 There is mounting evidence that D1 and D2 DA receptor subtypes are often colocalized on the same striatal neurons.120–123 Furthermore, striatal neurons projecting to GPi have been shown to send collaterals to GPe.124,125 Similar findings were reported in rats.126 These facts question the basic proposition of the Albin-DeLong model regarding the question of whether the two pathways from striatum to GPi are truly segregated and differentially affected by dopamine.84,127,128 According to the Albin-DeLong model, pallidotomies should alleviate akinesia, bradykinesia, and rigidity. However, the pallidotomy has been shown to be effective primarily in alleviating LID.90,129,130 This actually contradicts the logic of the model: removal of pallidal inhibition should, if anything, exacerbate dyskinesias.131 Finally, the Albin-DeLong model is a static model that attempts to explain akinesia and rigidity in terms of changes in tonic firing rates. However, the model fails to account for perhaps the most salient symptom of PD, the resting tremor. PD resting tremor is presumably related to changes in neuronal firing patterns and to the onset of oscillatory discharge in the CBG circuitry. This will be the focus of the next section. Firing patterns in the CBG circuit of primates are dramatically altered following MPTP treatment. There is an increase in the percentage of neurons that discharge in bursts. These bursts are either irregular or oscillatory and have been found in the STN, GPe, and GPi17,22–24,62,64,116 and in the primary motor cortex.15 Examples of these changes in firing patterns are depicted in Figure 6–2. The fact that the discharge of neurons in this system becomes oscillatory raises the question of whether and how this oscillatory activity is related to parkinsonian tremor. It seems obvious that if parkinsonism is marked by the onset of oscillatory discharge within the BG circuitry (often at the tremor frequency), then there must be a causal relationship to the concurrent appearance of parkinsonian tremor. However, it has been difficult to find a simple causal relationship between the two. Tremor induced in MPTP primates is at most intermittent and is therefore difficult to tackle experimentally. In addition, monkeys (even of the same species) do not always develop tremor, and when they do, some develop a low-frequency, 4 to 8 Hz tremor22 whereas others develop a 7 to 11 Hz or high-frequency tremor.17,23 Still others have a bimodal distribution of tremor frequencies in these ranges.24,132 The frequencies of neuronal oscillations in the BG and motor thalamus of MPTP-treated primates also have a bimodal distribution (even within a given neuron; see Fig. 6–3).22–24,62,133 Not surprisingly, then, some studies have reported that the neuronal oscillations often coincide with the episodes of tremor,22,62 whereas others have found that they do not.17,133 We can conclude, as recent studies have, that a correlation between tremor and neuronal oscillations is at most intermittent and is dynamic in nature.24,134 Neuronal oscillations at higher frequencies were found in human PD patients as well,135,136 indicating that a bimodal distribution of frequencies is not only characteristic of parkinsonian monkeys. We will return to this issue when we discuss the issue of neuronal synchronization in the next section. It is possible that the MPTP primate model does not replicate the true ongoing resting tremor exhibited by PD patients. Thus, it may be that the inability to find an obvious correlation between neuronal discharge and tremor in these monkeys results from some inadequacy of the model. However, MER studies in human PD patients have not clarified the issue either. These studies have found cells whose discharge is modulated in the tremor frequency range, called tremor cells, in the thalamus, in GPi, and in the STN.134–144 However, as in the primate studies, the issue of correlation with tremor is less than obvious. Several studies have looked at tremor cells in the GPi. Their results range from finding very little correlation of tremor cell discharge with tremor142,143 to at most intermittent coherence with tremor.134,144 With regard to tremor cells in the thalamus, although they exhibit a strong correlation with tremor, this may be the result of afferent feedback from the limbs, which would mean that these cells are not necessarily related to the source of the tremor. Similarly, one study claimed that tremor cells in the STN are synchronized with tremor, but only gave examples of this. The authors also pointed out that these tremor cells are usually sensitive to kinesthetic stimulation,133,141 suggesting again that the neuronal oscillations could result from afferent feedback. FIGURE 6–2 Discharge in the corticobasal ganglia circuit of MPTP-treated primates becomes bursty and oscillatory. Three extracellular traces from the globus pallidus of an African green monkey before (A) and after (B) MPTP treatment. FIGURE 6–3 Two frequencies in neuronal discharge of MPTP-treated primates. Spectrograms of spike trains from two simultaneously recorded units in the globus pallidus of an MPTP-treated African green monkey [lower plots in (A) and (B)]. The temporal average of these spectrograms is plotted above them. Note that the power shifts dynamically between two bands in the spectra (at ~7 and ~15 Hz) but is dominated by the lower one. In contrast, the “coheregram,” which measures the coherence (correlation coefficient in frequency space) between the units’ discharge as a function of time [lower plot in (C)], is dominated at all times by the higher frequency band. This suggests that the units are locked to some high-frequency network activity but discharge only intermittently (every other cycle on average). It should be noted that, in contrast to MER studies, other physiological studies in PD patients have found a temporal correlation between tremor and cranial macropotentials, such as surface potentials, local field potentials (LFPs), electroencephalography (EEG), and magnetoencephalography (MEG).145–149 The complex nature of synchrony between neuronal discharge within the CBG circuitry and parkinsonian tremors has generated a multitude of recent studies questioning whether PD tremors originate from a single oscillatory source within the CBG circuitry or from multiple oscillators. Most of these studies agree that there is a low correlation between the tremor of different body parts,150–154 indicating a weak coupling between the BG oscillators.132,134 Even though the relationship is complex, presumably because of the complex dynamical nature of CBG function, most researchers agree that tremor is related to oscillatory activity in this circuit. In recent years it has been hypothesized that the parkinsonian and dopamine agonists-induced symptoms result not only from changes in tonic firing rates of neurons but also from changes in their firing patterns.155–157 This issue has recently become the focus of several MPTP primate and human PD studies that are looking at the effect of administering dopamine agonists to the parkinsonian subjects. In these studies, the parkinsonian subjects were given levodopa, apomorphine (a nonselective ultra-fast D1 and D2 receptor agonist), or other selective DA receptor agonists. All of these drugs ameliorate the major symptoms of PD and can induce dyskinesias in humans and primates.158,159 As we shall see, dopamine replacement therapy does affect firing rates and firing patterns but not in a clear-cut and consistent way. In addition, the electrophysiological outcome of the treatment is highly dependent on whether the doses induce dyskinesias or not. In the absence of dyskinesias, levodopa has been shown in one study to lower firing rates in the GPi of MPTP primates, even below normal rates.64 However, in another study it has been shown to cause GPi neurons to increase, decrease, or exhibit no change in their firing rates.160 The firing rates in GPe of these monkeys were shown not to change following levodopa treatment.64 Our results indicate a reduction of GPi and an increase of GPe firing rates following “optimal” levodopa and bromocryptine therapy.61 In contrast, apomorphine seems to produce more consistent results: it lowers GPi firing rates in MPTP primates67 and in PD patients.161,162 The mean GPe firing rates of nondyskinetic PD patients who were given apomorphine increase.161 A single study claimed that the firing rate of striatal neurons in MPTP primates that were given apomorphine increases.67 However, it is unclear whether these cells were projection neurons or interneurons. When the dopamine agonist treatment causes dyskinesia, there seems to be a clear-cut effect on mean pallidal firing rates, independent of which drug is used. The rate of GPi cells of dyskinetic subjects (both MPTP primates and human PD patients) is dramatically reduced sometimes to an almost complete silence,66,136,160,161,163,164 and GPe firing rates increase.66,163,164 However, with respect to STN, the data are again inconclusive: one early study of PD patients rendered dyskinetic with apomorphine showed a decrease in STN firing rates,164 whereas another showed no change.136 A unique population of pallidal cells is located within the lamina surrounding the GPe and GPi. These cells, identified physiologically by their regular firing pattern and broad action potentials, are similar to the cholinergic neurons of the nucleus basalis of Meynert and are referred to as the pallidal border cells.165 An early study did not detect significant changes in the basal firing rates of these neurons after MPTP treatment.62 However, a reduction of their firing rates was observed following apomorphine treatment in the MPTP-treated monkey.163 A recent study found that the firing rates of pallidal border cells were significantly decreased after MPTP treatment (from 31 to 9 spikes/sec) and that this decrease was partially reversed (to 19 spikes/sec) by levodopa treatment.166 In the absence of dyskinesia, levodopa seems to increase slightly the number of bursting cells in the GPe and to decrease those in the GPi of MPTP-treated monkeys.64 However, when apomorphine was given to nondyskinetic PD patients, it caused no noticeable changes in firing patterns.161 In dyskinetic subjects, the effect of the DA agonist on firing patterns is even less clear. An early study noted a decrease in bursting discharge of GPe and GPi neurons in MPTP-treated monkey with apomorphine-induced dyskinesias.163 However, one study in these monkeys found that apomorphine did indeed decrease the number of bursting cells in the GPe but not in the GPi. Furthermore, a D2 receptor agonist seemed to reduce the number of bursting cells in both segments of the globus pallidus, whereas a D1 receptor agonist did so slightly only in the GPi.66 A recent study in PD patients demonstrated that although apomorphine induced dyskinesia and reduced the number of tremor cells in the GPi and STN, neurons in both these nuclei discharged with more bursts and less regularly.136 In summary, the neuronal responses to dopamine agonist treatment are variable: there are species and perhaps subject differences; different dopamine agonists have different effects167; and, finally, the responses depend on whether the drugs induce dyskinesia or not.66,160 The most salient and consistent effect of dopamine agonists on dyskinetic parkinsonian subjects is the excessive decrease in GPi output following their administration.61,66,156,156,160 Even if it seems hard to pinpoint precisely how, it is clear that the administration of dopamine agonists has a profound effect on firing patterns within the BG nuclei, as does DBS therapy.111,112 Up to this point we have regarded each structure in the CBG circuit as a single compartment and have neglected the spatial aspects of this circuit’s processing. However, anatomical evidence has shown that this circuit, depicted in Figure 6–1, is actually repeated over and over again. Retrograde and anterograde labeling studies have suggested that the CBG is comprised of five loops: the motor, the oculomotor, the limbic and two prefrontal loops.168,169 More recent studies tend to divide the basal ganglia into three domains: limbic, cognitive, and motor.170 There is an ongoing debate in the literature about whether and how these domains or loops interact with each other. Earlier studies have suggested that these loops are functionally segregated.168 Furthermore, there seems to be segregation at an even finer spatial scale within these loops.124,171–173 However, more recent anatomical studies have shown that these loops partially overlap and suggested a slight refinement of the scheme of strictly segregated loops within the CBG circuitry.174,175 Whereas the above studies emphasize the segregated aspects of CBG circuitry, other studies have highlighted the large degree of convergence found in these circuits.176,177 The gross anatomy of these circuits has revealed a funneling architecture wherein each structure in the feed-forward network—cortex, striatum, and pallidum—progressively decreases in size (in both volume and cell count). Furthermore, fine anatomical studies demonstrate the large degree of convergence and divergence present in these circuits.124,176,178,179 How can these seemingly opposing views of CBG circuitry—one of segregated loops and the other of a highly convergent and divergent network—be reconciled with each other? A careful calculation reveals that even in the presence of this great degree of convergence and divergence the probability that two pallidal neurons share 10 synapses is on the order of 0.1.155 This may not be enough to disrupt what may be largely segregated loops streaming through the CBG circuitry. Similarly, physically overlapping corticostriatal projections have been shown to have very few source and target neurons in common.180,181 It is possible that on a coarse spatial scale there exist segregated channels along the CBG circuit, each of which is comprised of convergent and divergent networks. In general, though, anatomical data can only reveal the upper limit of functional connectivity, as they do not reveal the true activity or physiological strength of synapses.114,182 MER in awake-behaving primates can provide the physiological data necessary to assess whether information flows through the BG in a segregated or shared manner. However, this usually requires the use of multiple electrodes.183 Even though it is possible to record more than a single neuron per electrode (depending on the electrodes’ impedance and on the density of neurons184), it is infrequent in the pallidum, and the results may be biased by spike-sorting limitations.185 Furthermore, by using multiple MER (mMER), it is possible to record distant neurons (~1–5 mm apart), which increases the chances of recording cells from functionally distinct CBG loops. We have used mMER extensively in the study of functional connectivity in the CBG circuits. One or two guide tubes, each containing four or eight glass-coated tungsten microelectrodes (0.2–1.2 MΩ at 1 kHz), are lowered into the brain. Each electrode is individually maneuvered to isolate cells whose extracellular activity is consequently recorded. The acquired waveforms are fed into an on-line template-matching device that can distinguish up to three spike waveforms per electrode. The use of two guide tubes enables the simultaneous recording of neurons in two distant structures simultaneously (e.g., substantia nigra and striatum or cortex and pallidum). A simple way to study functional connectivity is to use cross-correlation analysis of spike times.186–188 The times of spike emission are determined from the extracellular waveform using either on-line or off-line techniques.184 Given the spike times of two cells, the cross-correlogram is calculated as follows: for every lag shift τ, one counts the number of times a spike of the reference cells occurs at an interval τ from a spike of the trigger cell, with a precision of a given bin size h. This number is then normalized by the number of trigger spikes and by the bin size (h) to give the rate of the reference cell at time τ conditioned on the occurrence of a spike of the trigger cell at time 0.
Microrecording in the Primate MPTP Model
JOSHUA A. GOLDBERG, THOMAS BORAUD, AND HAGAI BERGMAN
The MPTP Primate Model of Parkinsonism
MPTP Toxicity
The MPTP Primate Model in PD Research
Tonic Neuronal Firing Rates in MPTP Primates and Their Impact on Neurosurgery
The Albin-DeLong Model of the Basal Ganglia
Ablative Surgery for Treatment of PD
Deep Brain Stimulation in PD Patients and MPTP Primates
Problems with the Albin-DeLong Model
Temporal Aspects of Neuronal Discharge in the MPTP Primate
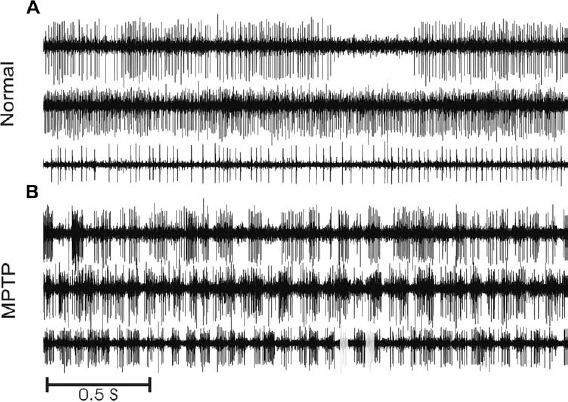
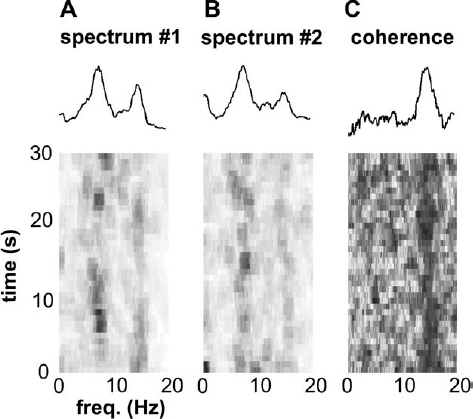
Oscillatory Discharge and Tremor
Dopamine Agonist Therapy and Induction of Dyskinesias
Dopamine Agonists and Neuronal Firing Rates
Dopamine Agonists and Neuronal Firing Patterns
Spatial Aspects of Neuronal Discharge in the MPTP Primate
Neupsy Key
Fastest Neupsy Insight Engine