2 Departments of Neurology and Psychiatry and Behavioral Sciences, University of Miami, Miller School of Medicine, USA
3 Department of Neurology, Herbert Wertheim College of Medicine, Florida International University, USA
Introduction
Most diseases go through a transitional stage (e.g. “pre-diabetes” and “pre-hypertension”) before they develop the clinical features that are required to fulfill established criteria for the diagnosis to be made with reasonable certainty. It is becoming increasingly important to introduce treatment for slowly progressive diseases at an earlier stage, so that treatment may have a greater impact and prevent or slow transition to more severe stages of the disease. Currently, disease-modifying treatments are not available for degenerative diseases of the brain, which are the main etiological factor for mild cognitive impairment (MCI) and dementia. Effective treatments are presently available to prevent cerebrovascular damage and interventions for such modifiable risk factors may modify the course of the disease, by preventing new vascular lesions from developing. Thus, secondary prevention remains the major option for providing a measurable therapeutic impact on the underlying disease, by earlier detection at the MCI (or even earlier) stage. The goals of this chapter, therefore, are to enable clinicians to: (a) recognize and diagnose MCI and its cognitive subtypes, (b) diagnose the likely etiology of MCI syndromes, (c) understand the risk factors for and modes of progression, and (d) be informed about optimal methods available for treatment and prevention of progression of MCI syndromes.
Definition
The term mild cognitive impairment was first described by Reisberg in 1982 as a condition associated with an increased risk of progression to dementia. It was, however, the Mayo Criteria for MCI published by Petersen et al[1,2]. which resulted in widespread use of this term. Originally designed for the diagnosis of a pre-Alzheimer disease condition, the term MCI is now widely used to describe the predementia phase of any disease that may ultimately progress to a dementia syndrome.
Grundman et al[3]. have proposed an adaptation of Petersen’s criteria, to improve reliability when these criteria are used in clinical trials, as follows:
2) abnormal memory functioning on the Logical Memory II subtest of the Wechsler Memory Scale
These adapted criteria may be operationalized for general clinical use, as shown in Table 6.1.
Mild cognitive impairment subtypes
The heterogeneity of MCI is a consequence of a number of factors, which include the methodology used to classify MCI, the underlying etiology of the MCI syndrome and the premorbid state of the patient, including the level of education, cultural background and the general medical, neurological, and psychiatric status. Broadly, MCI may be classified according to the predominant form of cognitive impairment (i.e. amnestic or non-amnestic MCI), the suspected etiology (e.g. Alzheimer’s disease, vascular cognitive impairment, Lewy body disease) or the progression rates of cognitive deficits (e.g. progressive versus non-progressive, or even reversible MCI). Amnestic and non-amnestic MCI may each be further subdivided into single or multiple domain MCI. Multidomain amnestic MCI (aMCI) requires impairment in memory and one or more non-memory domains; multidomain non-amnestic MCI (naMCI) requires impairment in two or more non-memory domains, such as attention/executive functioning, language, and visuospatial processing. Impairments in episodic memory occur not uncommonly in conjunction with other cognitive deficits. A purely amnestic form of MCI occurs in approximately one-third of MCI cases, and the largest single MCI group is characterized by impairment in memory and one or more non-memory domains.
Table 6.1 Criteria for amnestic mild cognitive impairment
| Formal criteria | Operationalization of formal criteria in clinical practice |
| Memory complaint, preferably corroborated by an informant | Interview with informant |
| Objective memory impairment for age and education | Score below age and education cut-off on neuropsychological test (e.g. logical memory II subscale [delayed paragraph recall] from the Wechsler Memory Scale) |
| Largely intact general cognitive function | Mini-Mental State Examination score between 24 and 30 (inclusive) |
| Essentially preserved activities of daily living | Cognition and functional capacity not sufficiently impaired for a diagnosis of dementia |
| Not demented | Clinical Dementia Rating of 0.5 |
Reproduced from Petersen et al[4]. with permission from Lippincott Williams and Wilkins.
Longitudinal studies in memory disorder clinics have demonstrated a rate of progression from MCI to Alzheimer’s disease (AD) of 12–15% per year, with a low rate of reversal (less than 5% per year) while community-based epidemiological studies demonstrate a lower rate of conversion (5–10% per year) and higher rates of reversal (up to 25% per year). These findings suggest that factors other than cognitive test performance per se, such as subtle impairment of functional ability observed by family members and friends or colleagues at work, are a strong determinant of the future rate of progression.
Pathophysiology
A transitional, predementia state, between normal cognition and established dementia, is likely to exist for most recognizable dementing disorders The use of different criteria for diagnosing MCI may result in MCI prevalence rates of 1–3% of the population over 65 years of age. Nevertheless, regardless of the criteria used, patients diagnosed with MCI have been found to have a high likelihood of progression to dementia and often to Alzheimer’s disease.
The most well-studied pathological entity that results in an MCI syndrome is AD. Pathological changes associated with this disease may be present a decade or more before the onset of clinical symptoms and signs, and include the deposition of amyloid beta protein in the neocortex. Although amyloid deposition itself may result in subtle cognitive deficits, it is the neurodegenerative phase of AD which results in the deficits in delayed memory characteristic of this disease. Deficits on delayed recall tests are caused by neurodegenerative involvement of the medial temporal structures such as the entorhinal cortex (ERC) and the CA1 sector and subiculum of the hippocampus (HP).
The plasticity of the brain and the effects of cognitive reserve (see below) allow some patients with advanced pathological stages of the disease (i.e. Braak stage V or VI) to escape cognitive symptoms and functional deficits. In fact, in one study about 30% of individuals who met neuropathological criteria for AD at autopsy were considered non-demented during life. In another longitudinal study, almost all patients classified as MCI during life were found to have neurodegenerative pathology at autopsy. In about 30% of these cases, pathologies other than AD, such as Lewy bodies, argyrophilic grain disease, hippocampal sclerosis and/or cerebrovascular disease, are present.
 SCIENCE REVISITED
SCIENCE REVISITEDThe concept of vascular cognitive impairment (VCI) has evolved over several decades as advancements in brain computed tomography (CT) and especially magnetic resonance imaging (MRI) have allowed greater detection of vascular damage. Because vascular disease is prevalent in older people , vascular lesions are often co-mingled with Alzheimer pathology. The concept of VaMCI has also evolved because subclinical vascular damage, such as white matter lesions, infarcts, and microhemorrhages, have been found to associate with cognitive consequences, even in the absence of a clinical event, such as a stroke. Evidence from large population-based studies has shown that subclinical vascular damage is associated with a spectrum of cognitive deficits ranging from subtle changes to MCI (manifesting often as executive dysfunction, rather than an amnestic disorder) to dementia.
Vascular insults are an important cause of and contributor to the development of VaMCI, although the volume and location of tissue both necessary and sufficient to cause such impairment have not been established. For example, small “strategic” infarcts in the anterior thalamus and other areas can produce amnestic disorders of varying severity, and small infarcts in other locations can produce deficits corresponding to the functions attributable to those regions. However, both the volume and the number of infarcts are known to be associated with the risk of dementia. Likewise, the volume of white matter lesions necessary to cause VaMCI or VaD is unknown. However, community-based data show that both Alzheimer and vascular pathology are prevalent and that each contributes to cognitive performance in those with MCI that reach autopsy.
The presence, severity, and type of cognitive impairment in a given individual depend not only on the location, severity, and type of pathology present in the brain, but also on the resilience of that individual to withstand the effects of that pathology. This resilience is known, broadly, as cognitive reserve and is associated with various factors that can be subdivided as “pure” cognitive reserve and “brain reserve.” Among those factors related to pure cognitive reserve are the individual’s level of education, overall intelligence, occupational attainment, and exposure to information as well as exposure to various stimuli and activities. Factors associated with brain reserve include brain size, age, developmental and premorbid conditions, such as dyslexia and attention deficit disorder, brain trauma, cerebrovascular disease, and systemic illnesses. It is importance to consider cognitive reserve as a major factor determining when and with what severity an individual with a specified brain pathology, such as Alzheimer’s disease, will manifest with cognitive symptoms and objective evidence of impairment.
Symptoms mimicking early dementia and impaired performance on cognitive tests may be related to a variety of medical conditions, effects of medications, psychosocial factors, and psychiatric conditions, such as anxiety, depression, and personality disorders. Furthermore, in the early phase of MCI, it may be difficult to distinguish (a) functional impairment associated with normal aging from abnormal aging, especially in the presence of age-related conditions such as arthritis, visual and hearing impairment, and (b) cognitive deficits from the normal age-related cognitive decline, especially among those with high or low cognitive reserve, psychiatric co-morbidity and those with visual or hearing impairments.
 TIPS AND TRICKS
TIPS AND TRICKSNeuropsychological assessment
Neuropsychological evaluation is considered an important tool for confirming the diagnosis of MCI and when it is easily available and affordable, neuropsychological assessment is the preferred method of diagnosis. Many clinicians who conduct dementia and cognitive evaluations use combinations of convenient and brief cognitive tests to make an MCI diagnosis. While simple cognitive screening tests, such as the Folstein Mini-Mental State Examination, are useful for distinguishing dementia from a normal cognitive state, they remain insensitive for detecting MCI. Cognitive screening tests that may better distinguish MCI from normal cognition include the Montreal Cognitive Assessment (MoCA) and the Multiple Delayed Recall Test.
 TIPS AND TRICKS
TIPS AND TRICKSNeuropsychological tests commonly used to determine performance in domains required to assess MCI include verbal fluency measures, such as the Controlled Oral Word Association Test (COWAT). Various cut-off scores have been used for these tests, although 1.5 SD below age- and education-adjusted norms, for a single measure, appears to be most effective in identifying MCI. It is also important to consider the ethnic and cultural background of subjects in determining appropriate cut-off scores. The use of multiple measures to identify memory impairment has been suggested to improve reliability. A cut-off score of at least 1.0 SD below mean normative values on at least two cognitive tests in the same cognitive domain is recommended to decrease the false-positive rate in classification.
There appears to be considerable overlap between amnestic and non-amnestic MCI, depending on the criteria and cut-off scores used to classify impairment. The prevalence rates for MCI subtypes will depend on the use of different cut-off scores, the choice of individual tests used to identify impairment and the control group used to derive such cut-off scores. Reducing the threshold for classifying impairment in a memory test from 1.5 to 1.0 SD will have the effect of increasing the frequency of aMCI relative to naMCI. Individuals with high premorbid educational attainment, or cognitive reserve, may be able to compensate for their deficits because of their greater knowledge base and familiarity with the test-taking process and by employing various strategies that allow them to perform well on cognitive measures, in spite of their deficits. Those with low educational levels may perform far worse than expected, not only because of a lower knowledge base but also because of lack of familiarity with test taking and the associated anxiety and attentional problems.
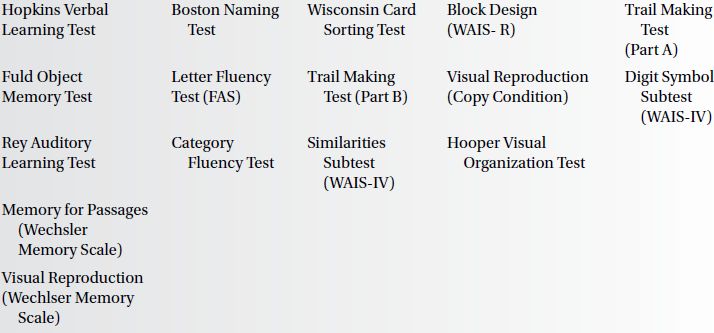
The cognitive assessment of MCI requires the assessment of memory, language, visuospatial skills, and executive function. Suggested measures for each of these domains are presented in Box 6.1.
Biomarkers in mild cognitive impairment
Biomarkers are surrogates of underlying pathology and unlike clinical and neuropsychological assessments, are not subject to influence by various demographic, psychosocial, medical, and psychiatric factors, nor by hearing and visual deficits. An individual may be biomarker positive without necessarily manifesting any cognitive symptoms or deficits. Biomarkers can be useful in diagnosis by identifying the presence of underlying pathology, even in the preclinical stage of diseases such as AD and FTLD. The accumulation of amyloid (Abeta1-42) in the brain suggests the first phase of AD pathology, whereas regional brain atrophy suggests the presence of neurodegenerative pathology in the brain. Besides being useful diagnostically, biomarkers may also predict the rate of progression of the clinical syndrome, because the severity of the underlying pathology, as measured by the biomarker, is often correlated to the rate of clinical progression.
Cerebrospinal fluid (CSF)
Currently the most promising CSF biomarkers for the diagnosis of early AD are the ratio of CSF Abeta levels to tau protein and CSF phosphotau -231 (ptau-231). CSF Abeta1-42 (the 42 amino acid form of Abeta) is an early marker (including the preclinical and MCI stages) of the amyloid phase of the disease. Somewhat paradoxically, CSF Abeta1-42 is lower in individuals with Abeta pathology, starting in the preclinical phase. CSF total tau, i.e. T-tau, is associated with tangle formation and phosphotau is a marker of the later neurodegenerative phase of the disease. Both tau and phosphotau are elevated in CSF of individuals with AD brain pathology. The CSF Abeta-42:tau ratio differentiated patients with subjective cognitive complaints, with naMCI, and with aMCI from healthy controls. CSF biomarkers have also been shown to have utility in predicting cognitive decline in cognitively normal older adults and progression to a MCI state, as well as progression of aMCI to AD. The measurements are commercially available but their use for predictive purposes in asymptomatic or even MCI patients is currently confined to the research setting.
Neuroimaging
Alterations in the structure of the brain can be detected and quantified by several structural imaging techniques, such as computed tomography (CT) and magnetic resonance imaging (MRI), especially in the medial temporal lobes where AD- and FTLD-related degenerative pathology appear to be most prominent early in the disease process. Functional changes in the brain can be assessed with positron emission tomography (PET) and single photon emission computed tomography (SPECT), as well as by functional MRI (fMRI). Amyloid deposition in the brain can be detected using investigational PET scans with either C-11 or F-18 labeled ligands that bind to fibrillar amyloid beta protein. Although FDA approval of amyloid PET imaging appears to be imminent, practice guidelines are not yet established for using these scans for diagnosis or prognosis in MCI patients. Genetic markers, such as APOE genotypes, can identify subgroups of individuals who are at elevated risk for cognitive decline and the development of AD pathology but the value of APOE genotyping for prognosis in individual subjects with MCI (i.e. in clinical practice) remains to be established.
Magnetic resonance imaging
The presence of an underlying pathology that may be related to an MCI syndrome is currently best identified using MRI, because of its high spatial resolution and ability to distinguish tissue types based on biochemical constituency. The absence of pathology and its presence are important in making an accurate clinical diagnosis, although in many age-related diseases, such as vascular or degenerative dementia, non-specific pathological processes may be discovered, which may obfuscate the diagnosis. The most frequent clinical questions that can be answered by MRI include whether or not the findings on the brain images exclude certain diagnoses, such as vascular dementia, normal pressure hydrocephalus (NPH), space-occupying lesions, such as chronic subdural hematoma or a brain tumor, miscellaneous lesions such as amyloid angiopathy and diseases of the white matter. However, the diagnosis of AD, in its MCI or dementia phase, is the most frequent diagnostic entity that clinicians must address, because the prior probability that patients have this condition is relatively high and the most common query from patients or their relatives is whether or not there is any evidence of AD.
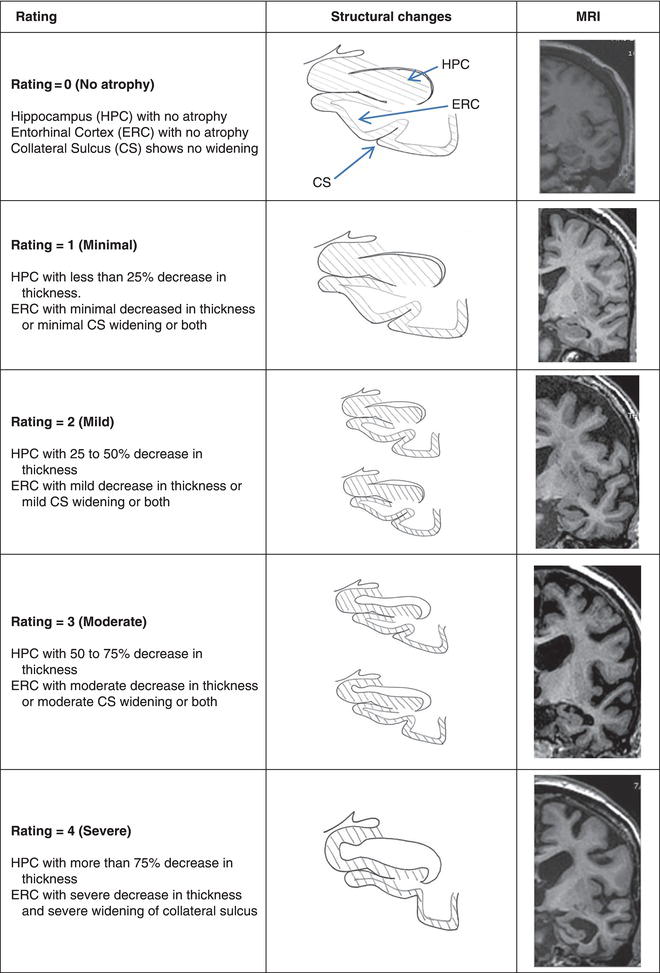
Assessment of hippocampal and entorhinal cortex atrophy in structural brain images could be an inclusive test for the diagnosis of prodromal and probable AD. The pathological process in AD begins in medial temporal structures and the density of this pathology has a proportional effect on the degree of atrophy in these structures. Both hippocampal and entorhinal cortex atrophy, as measured by MR imaging, are markers for AD-related pathology among MCI patients. Atrophy of these structures can be measured semi-quantitatively by the clinician using a visual rating scale (Figure 6.1). The severity of atrophy is correlated with the risk for cognitive impairment and for the rate of progression[5], although the onset and progression of the cognitive deficits are also dependent on cognitive and brain reserve capacity, including the presence of vascular brain disease. In the presence of an MCI syndrome of apparently degenerative etiology, the absence of hippocampal and especially entorhinal cortex atrophy suggests an alternative to AD, such as Lewy body dementia, as the etiology.
Vascular and degenerative diseases are very common among the elderly, so it should be expected that MRI evidence of both pathologies will co-exist in many patients. However, in any given case the individual contributions of degenerative and vascular disease to the cognitive impairment may not be easily discernible. Except for cardiac arrest and hypoxic-ischemic encephalopathy, the extent of hippocampal and entorhinal cortex atrophy as a result of pure vascular brain disease should be minimal. Cognitive impairment resulting from vascular brain disease is related not necessarily to the volume of the brain involved by infarctions but to the location of these infarctions.
Figure 6.1 Visual rating scale. CS, collateral sulcus; ERC, entorhinal cortex; HPC, hippocampus. Reproduced from Urs et al[2]. with permission from Lippincott Williams and Wilkins.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



