Chapter E44 Minimal Access Skull Base Approaches
Endoscopic Endonasal Approaches
Historical Background
Since the 1980s, several ENT surgeons pioneered the functional endoscopic sinus surgery (FESS),1–5 thus encouraging the “pure” endoscopic endonasal approach to the sella,6 performed first by Jankowski;7 then Jho and Carrau extended this technique,8–10 followed by other teams.11–14 These experiences supported a continued structured evolution of the endoscopic technique: new skills have been transferred across subspecialties, new instruments have been designed,15–18 new image-guidance systems have been fashioned,19–24 and new surgical corridors have been defined, thus disclosing the way to the pure endoscopic approach to lesions affecting the skull base, from the frontal sinus to the C2 body.14,25–28 The shift toward minimally invasive procedures represents the evolution of previous views: Ketcham’s concept of craniofacial resection29 evolved in the concept of “endoscopic craniofacial resection,”30 and Apuzzo’s inspired guess of using the endoscope in transcranial surgery31 has been translated in Kassam’s paradigm of “360 degree surgery,” which suggests the use of an endoport to unlock intracranial lesions.32
Classification
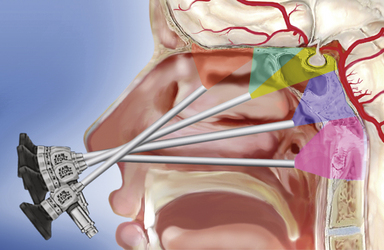
The sphenoid sinus is in the center of the skull base and the starting point for many extended endonasal approaches (Table E44.1) because critical anatomical structures, such as the internal carotid artery and the optic nerve, are identified here and then followed to other areas.14,25–27,33–36 The midline ventral skull base can be exposed from the frontal sinus to the body of C2 through specific modules: transcribriform, transtuberculum, transclival, and transodontoid25,37,38 (Fig. E44.1). Depending on the lesion extension along the sagittal plane,14,26,28 contiguous modules can be combined, and the lateral boundaries of each module are dictated by critical neurovascular structures. Lesions originating in or extending to paramedian areas, such as the cavernous sinus, the lateral recess of the sphenoid sinus, and the pterygopalatine fossa, can be approached through a transethmoid transantral transpterygoid route,39–42 and several paramedian modules have been developed to improve the endonasal exposure of the orbit, the petrous apex, the infratemporal fossa, and the jugular foramen.27,30,43–45
TABLE E44.1 Pure Endoscopic Approaches to the Skull Base
| Endoscopic Approach | Surgical Target |
|---|---|
| Endoscopic Endonasal Approaches | |
| Midline Approaches | |
| Transcribriform | Cribriform plate |
| Transtuberculum transplanum | Suprasellar area Third ventricle |
| Standard transsphenoidal | Sellar, suprasellar, and parasellar areas |
| Transclival | Clivus Interpeduncular and prepontine areas Prepontine area |
| Transodontoid | Craniovertebral junction Odontoid Superior cervical spine (C2 body) |
| Paramedian Approaches | |
| Supraorbital and transorbital | Orbital roof Medial intraconal space |
| Transmaxillary transpterygoid | Pterygopalatine fossa Lateral recess of the sphenoid sinus Lateral compartment of the cavernous sinus Meckel’s cave Infratemporal fossa Medial aspect of the petrous apex Jugular foramen |
| Endoscopic Transcranial Approaches | |
| Supraorbital | Anterior skull base Middle skull base |
| Retrosigmoid | Cerebellopontine angle |
Imaging Techniques
Patients are studied with both magnetic resonance imaging (MRI) and computed tomography (CT), which are complementary in delineating the individual anatomy of patients harboring skull base lesions, thus optimizing patient selection and operative procedure.35,46,47
 Identification of septal deviation, crest, or polyps claiming adequate correction during the same operative time
Identification of septal deviation, crest, or polyps claiming adequate correction during the same operative time
 Configuration of the paranasal sinuses such as pneumatization of sphenoid sinus, insertion of the sphenoid septa, presence of any Onodi (sphenoethmoid) cell48
Configuration of the paranasal sinuses such as pneumatization of sphenoid sinus, insertion of the sphenoid septa, presence of any Onodi (sphenoethmoid) cell48
 Bone dehiscence at the level of the optic and carotid prominences, dorsum sellae, and intrapetrous carotid canal
Bone dehiscence at the level of the optic and carotid prominences, dorsum sellae, and intrapetrous carotid canal
 Morphology of the olfactory cleft and insertion of the basal lamella and of the uncinate process
Morphology of the olfactory cleft and insertion of the basal lamella and of the uncinate process
 Vidian canal on axial and coronal images and its relation with the foramen lacerum
Vidian canal on axial and coronal images and its relation with the foramen lacerum
 Configuration of the craniovertebral junction
Configuration of the craniovertebral junction
 Nasopalatine line, which represents the inferior limit of the endonasal exposure
Nasopalatine line, which represents the inferior limit of the endonasal exposure
Preoperative CT angiography is used for intraoperative image guidance.
 Three-dimensional configuration, vascularity, and consistency of the lesion
Three-dimensional configuration, vascularity, and consistency of the lesion
 Relationship of the lesion with the neurovascular structures
Relationship of the lesion with the neurovascular structures
 Intactness of the arachnoidal plane and carotid sheath
Intactness of the arachnoidal plane and carotid sheath
 Infiltration of the brain, hypothalamus, or brainstem and perineural spreading
Infiltration of the brain, hypothalamus, or brainstem and perineural spreading
 Interpretation of signal from tumoral, scar, inflammatory, and adipose tissue
Interpretation of signal from tumoral, scar, inflammatory, and adipose tissue
 Critical anatomical variations, such as “kissing carotid arteries” or aneurysms
Critical anatomical variations, such as “kissing carotid arteries” or aneurysms
Surgical Technique49
Perioperative antibiotic prophylaxis with a broad-spectrum cephalosporin50 is administered half an hour before surgery and continued for 5 days. We do not usually insert any lumbar drainage. Neurophysiological monitoring of brain and nerve function allows detection of cerebral ischemia and prevention of cranial nerve injury. An optimal anesthesia and analgesia combination51 contributes to a dry field, will improve surgical precision, and can reduce complications and frustration during the surgical procedure.
Cotton pledgets soaked in diluted (1:1) povidone-iodine solution are gently slipped into the nasal cavities and the nose and upper lip are disinfected.
The procedure is performed with a rigid 0-degree endoscope, 18 cm in length, 4 mm in diameter (Karl Storz & Co., Tuttlingen, Germany), without any working channel and inserted in an irrigation shaft to keep the lens clean. A two-nostrils four-hands technique14,26,27,52,53 is recommended, with the endoscope used freehand during the whole procedure; it is held by the surgeon’s nondominant hand and, after the anterior sphenoidotomy, by the surgeon’s assistant. A 30-degree or 45-degree angled scope increases the angle of view when a more lateral view is needed, quite often in the intradural phase of the procedure.
Standard Approach
The surgical corridor between the middle turbinate and the nasal septum is enlarged by pushing the middle turbinate laterally, after topical decongestion with cotton pledgets soaked with proper anesthetics. The endoscope is moved, parallel to the nasal floor, toward the choana and angled up along the sphenoethmoid recess. After anterior sphenoidotomy, the identification of the anatomical landmarks54 over the posterior wall of the sphenoid guide the opening of the sellar floor. Dura opening is tailored to the lesion extension.
Microadenomas can be removed en bloc or piecemeal, depending on their consistency and the presence of a pseudocapsule.55 Intra- and suprasellar macroadenomas are removed systematically, starting from the bottom of the sella, because early descent of the stretched suprasellar cistern may hide tumor remnants.52 Tumor extension in the medial compartment of the cavernous sinus can be removed with atraumatic curets and suction cannulas. Cystic lesions as well as pure intrasellar craniopharyngiomas are well established indications for this technique.56
Extended Approaches
The idea of an approach extending beyond the sella should be credited to Hardy,57 and the routine use in clinical practice is due to Martin Weiss.58 This first step of the surgical procedure can be considered the common denominator of all the extended approaches. The endoscope is inserted in the right nostril, which is stretched upward while instruments run below it. Essential aspects of this stage, according to Kassam and his group,27 include the following:
 Middle turbinectomy, usually unilateral, with lateralization of the middle turbinate in the other nostril, provides the adequate room to access and manage confortably the lesion in most cases.
Middle turbinectomy, usually unilateral, with lateralization of the middle turbinate in the other nostril, provides the adequate room to access and manage confortably the lesion in most cases.
 Harvesting of the nasoseptal flap is done (see later discussion under “Reconstruction”).
Harvesting of the nasoseptal flap is done (see later discussion under “Reconstruction”).
 A wide anterior sphenoidotomy is the next step.
A wide anterior sphenoidotomy is the next step.
 A posterior septectomy permits instruments coming from the two nostrils to work without conflict.
A posterior septectomy permits instruments coming from the two nostrils to work without conflict.
 The removal of posterior ethmoidal cells completes the exposure of the posterior wall of the sphenoid sinus with its anatomical landmarks.
The removal of posterior ethmoidal cells completes the exposure of the posterior wall of the sphenoid sinus with its anatomical landmarks.
Transcribriform Approach
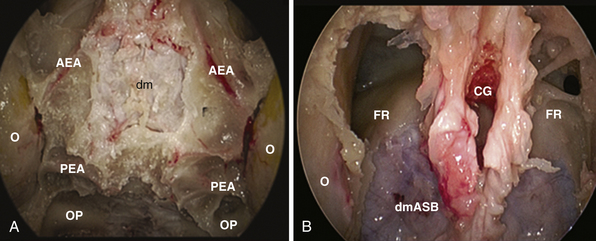
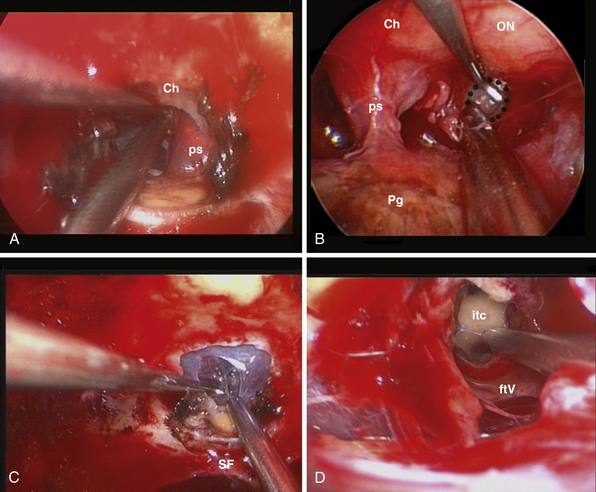
This approach is used for the management of cerebrospinal fluid (CSF) leaks,59 meningoencephaloceles, esthesioneuroblastoma,30,60 and meningiomas.61–64 It is limited by the medial orbital wall laterally, but it can be expanded along the sagittal plane, depending on the lesion extension. However, to expose the cribriform plate, the posterior limit is marked by the posterior ethmoidal artery (Fig. E44.2A). The exposure can be further expanded anteriorly, toward the frontal sinus, through a transfrontal module, which provides access to its floor and the posterior wall30 (Fig. E44.2B).
The main steps to expose the midline anterior skull base are as follows:65
 Bilateral anterior and posterior ethmoidectomy: entering the ethmoid bulla inferomedially, the lamina papyracea comes into view and should be preserved during the ethmoidectomy.
Bilateral anterior and posterior ethmoidectomy: entering the ethmoid bulla inferomedially, the lamina papyracea comes into view and should be preserved during the ethmoidectomy.
 Exposure of the frontal recess and of the anterior ethmoidal artery: opening the agger nasi air cell highlights the posterior wall of the frontal recess, which leads to the anterior ethmoid canal. The lamina papyracea is fractured with a blunt instrument and lateralization of the periorbit highlights the artery, where it leaves the orbit to enter its osseous canal. At this level, the anterior ethmoidal artery can be easily bipolar coagulated. The frontal recess guides toward the frontal sinus floor, which can be removed to enlarge the exposure anteriorly.
Exposure of the frontal recess and of the anterior ethmoidal artery: opening the agger nasi air cell highlights the posterior wall of the frontal recess, which leads to the anterior ethmoid canal. The lamina papyracea is fractured with a blunt instrument and lateralization of the periorbit highlights the artery, where it leaves the orbit to enter its osseous canal. At this level, the anterior ethmoidal artery can be easily bipolar coagulated. The frontal recess guides toward the frontal sinus floor, which can be removed to enlarge the exposure anteriorly.
 Identification and coagulation of the posterior ethmoidal artery: its bony canal runs in the ethmoidal roof horizontally and is located at an average of 8.3 mm (range 6-11 mm) anterior to the optic nerve exit from the optic canal.65
Identification and coagulation of the posterior ethmoidal artery: its bony canal runs in the ethmoidal roof horizontally and is located at an average of 8.3 mm (range 6-11 mm) anterior to the optic nerve exit from the optic canal.65
 Opening of the ethmoidal plate with bone punches or microdrill.
Opening of the ethmoidal plate with bone punches or microdrill.
In olfactory groove meningiomas, the dura is coagulated and opened along the midline. In lesions extended to the crista galli, the dura is cauterized and incised lateral to the insertion of the falx; after coagulation of the anterior falcine artery and the inferior sagittal sinus, the falx is transected and the dura is mobilized anteriorly. Further anterior extension of the lesion may require drilling of the crista galli, which can be hyperostotic or pneumatized (10% of cases).66 After internal debulking, extracapsular dissection proceeds in a lateral to medial direction; when anterior cerebral artery branches or the anterior communicating artery lie on the surface of the tumor, a piecemeal removal is performed and the last tumor remnants are carefully dissected.
In esthesioneuroblastomas extending intradurally, the olfactory bulbs are dissected from the surface of the brain and remain attached to the dura; focal areas of brain invasion are removed by suction and dissection. The olfactory nerves are then transected posteriorly and dura is removed completely; additional dural margins can be excised for frozen section analysis.60
Transtuberculum-Transplanum Sphenoidale Approach
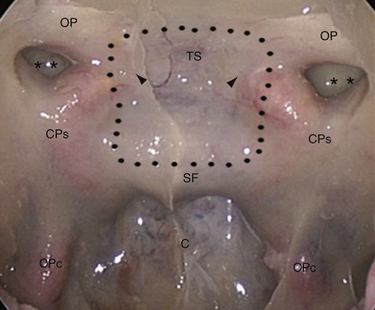
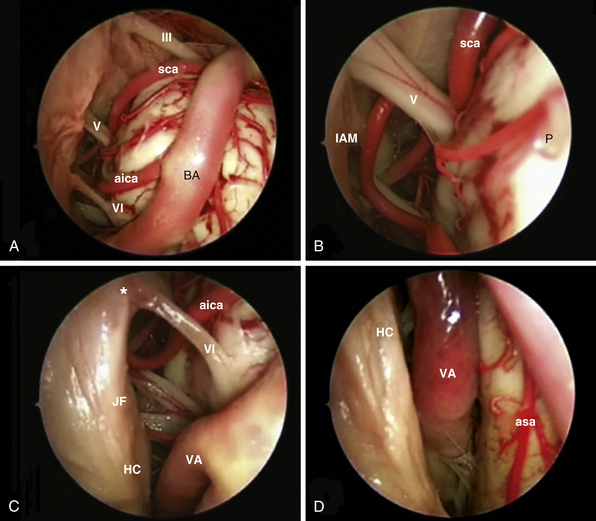
In 1980 Ed Laws, Jr., described the microscopic transsphenoidal approach to suprasellar lesions by taking down the planum sphenoidale,67 and the same approach was adapted for endoscopy.67,68 It is bordered by the optic nerve’s bony prominence, the opticocarotid recess, and the bony prominence of the parasellar segment of the internal carotid artery (Fig. E44.3).
The main aspects of this module are as follows:
 Opening of the sellar floor and drilling of the tuberculum are done in order to widen the caudal-cranial angle of exposure of the third ventricle and retrosellar area.
Opening of the sellar floor and drilling of the tuberculum are done in order to widen the caudal-cranial angle of exposure of the third ventricle and retrosellar area.
 Management of the superior intercavernous sinus with its isolation is done with two horizontal incisions and bipolar coagulation, in case of craniopharyngiomas, and by coagulation in meningiomas.
Management of the superior intercavernous sinus with its isolation is done with two horizontal incisions and bipolar coagulation, in case of craniopharyngiomas, and by coagulation in meningiomas.
 Lateral extension of the bone window: the medial opticocarotid recess lies at the point of maximum contact between the internal carotid artery and the optic nerve and corresponds intracranially to the middle clinoid process.69 Bone removal in this critical area may be refined with a Kerrison rongeur or an ultrasonic bone curet.70
Lateral extension of the bone window: the medial opticocarotid recess lies at the point of maximum contact between the internal carotid artery and the optic nerve and corresponds intracranially to the middle clinoid process.69 Bone removal in this critical area may be refined with a Kerrison rongeur or an ultrasonic bone curet.70
 Removal of the sphenoid planum: in order to preserve olfaction, the posterior ethmoidal artery marks the anterior limit of the exposure and the rostral margin of the nasal septum (1 to 2 cm) is left intact.
Removal of the sphenoid planum: in order to preserve olfaction, the posterior ethmoidal artery marks the anterior limit of the exposure and the rostral margin of the nasal septum (1 to 2 cm) is left intact.
Once the dura has been opened, the following factors are evaluated:
In many cases, mainly cystic retrosellar lesions, removal of the whole sellar floor, flush to the clivus, provides a certain degree of pituitary mobility and the parapituitary stalk corridor could be enough to expose the prepontine cistern. When dealing with solid intrasellar lesions, a transpituitary route or additional pituitary mobilization is considered (Fig. E44.4). The pituitary gland can be mobilized on one side or reflected upward. Kassam has described an en bloc transposition.69 In order to reduce stretching of the infundibulum and the length of this step, a selective dislocation upward of the central part of the gland, through a V-shaped cut of the gland, may be considered.
Tumor removal is tailored to the lesion, following the microsurgical principles.71–74
In suprasellar supradiaphragmatic craniopharyngiomas (Fig. E44.5) the surgical strategy is guided by their relationship with the infundibulum, as has been proposed by Kassam and associates.74–76 In prechiasmatic lesions the tumor is seen immediately after dural opening, with the optic nerve and chiasm stretched superiorly. During extracapsular dissection, the distal branches of the superior hypophyseal artery, which supply the inferior aspect of the chiasm and the pars tuberalis of the pituitary stalk, should be preserved. Retrochiasmatic craniopharyngiomas can be unlocked through intra- or parapituitary stalk routes, depending on their pattern of growth. When the lesion expands in the infundibulum, the intrapituitary stalk corridor leads toward the third ventricle chamber.
 The chiasm may lie in a prefixed position, just behind the dura, which should be opened carefully above the sella without transgressing the arachnoid.
The chiasm may lie in a prefixed position, just behind the dura, which should be opened carefully above the sella without transgressing the arachnoid.
 During tumor dissection the ventricular floor should never be violated at the level of the mammillary bodies, or even more posteriorly, to avoid brainstem injury.
During tumor dissection the ventricular floor should never be violated at the level of the mammillary bodies, or even more posteriorly, to avoid brainstem injury.
 During tumor dissection along the lateral walls, adherent gliotic or invasive finger-like tumoral tissue at the level of the hypothalamus should not be violated.
During tumor dissection along the lateral walls, adherent gliotic or invasive finger-like tumoral tissue at the level of the hypothalamus should not be violated.
Retroinfundibular craniopharyngiomas can be unlocked, preserving the pituitary stalk, using the parapituitary stalk corridors and by mobilizing the gland on one side or upward, as proposed by Kassam; the critical point of this step is to avoid stretching of the pituitary stalk and retrograde cell death in the hypothalamus. When required, the surgical window can be further enlarged by drilling the dorsum sellae and removing the posterior clinoids.69
Tuberculum sellae meningiomas can be devascularized early and completely removed, together with the dura and bone involved.64,77,78 Internal debulking can be performed with radiofrequency monopolar wire electrodes (SurgiMax; Elliquence International, Hewlett, NY) and Cavitron ultrasonic aspirator.37,61 In the extracapsular dissection the pituitary gland, the pituitary stalk, and the chiasm are identified and protected with a cottonoid, with sparing of the perforators to the visual apparatus. The optic chiasm can present a neurovascular conflict with the precommunicating segment of the anterior cerebral artery.78
Transclival Approach
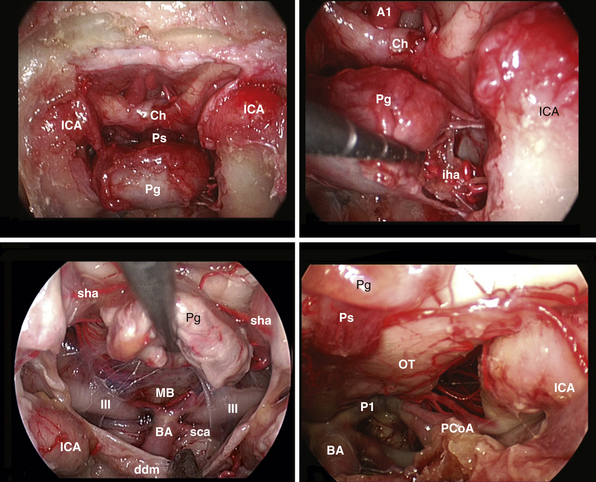
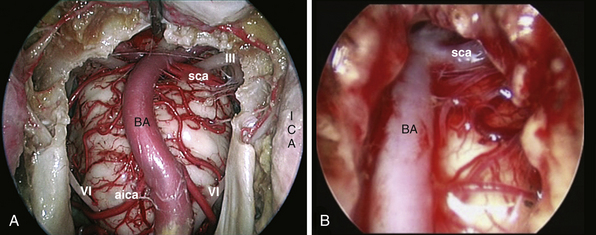
The clivus extends from the posterior clinoids to the craniovertebral junction (Fig. E44.6). Depending on the lesion extension along the cranial-caudal axis, the transclival approach is carried out across three main corridors—superior, middle, and inferior—which can be plainly combined as well.26,27,79–85 The superior corridor unlocks the retrosellar area and has been described in the previous module. The middle corridor is developed through the following steps:
 Lateral dissection of the mucosa covering the vomer: in a medial to lateral direction, the vomer-sphenoid junction, the ventral end of the palatovaginal canal, and the pterygoid canal are identified.
Lateral dissection of the mucosa covering the vomer: in a medial to lateral direction, the vomer-sphenoid junction, the ventral end of the palatovaginal canal, and the pterygoid canal are identified.
 Drilling of the sphenoid floor: the lateral limit is the pterygoid canal, which points toward the anterior genu of the intrapetrous carotid artery.84
Drilling of the sphenoid floor: the lateral limit is the pterygoid canal, which points toward the anterior genu of the intrapetrous carotid artery.84
Extradural lesions, such as chordomas, have a propensity for insinuation into bone along lines of least resistance, and careful exploration at the lateral borders of the clivectomy is mandatory. At this level the abducens nerve is at risk at the level of its dural porus (Dorello’s point), which is on average situated 1 cm from the midline and 20 mm below the posterior clinoid process.86 At this level the nerve is in close relationship with the dorsal meningeal artery. Bleeding from the basilar venous plexus is managed.87
The intradural extension of chordomas and meningiomas is unlocked through a midline dura opening.81
After tumor removal, intradural exploration highlights, on the midline, the pons, the medulla oblongata, and the basilar artery (Fig. E44.7). Running the endoscope above the cisternal segment of the abducens nerve, the cisternal segment of the trigeminal nerve can be followed toward its dural porus, which lies about 5 mm above and lateral to Dorello’s point. Running the endoscope below the abducens nerve, the anteroinferior cerebellar artery can be followed toward the acoustic-facial bundle and, just below it, the lower cranial nerves entering the jugular foramen, which lies about 14 mm lateral to Dorello’s point. The posteroinferior cerebellar artery or its branches can be found anterior to the lower cranial nerves.
The inferior corridor unlocks the craniovertebral junction through the following steps:
 The vomer is drilled; it should be flush with the hard palate.
The vomer is drilled; it should be flush with the hard palate.
 C-shaped incision of the mucosa of the rhinopharynx: its blood supply from the ascending and greater palatine and the ascending pharyngeal arteries should be preserved.
C-shaped incision of the mucosa of the rhinopharynx: its blood supply from the ascending and greater palatine and the ascending pharyngeal arteries should be preserved.
 Lateralization of longus colli and longus capitis muscles.
Lateralization of longus colli and longus capitis muscles.
 Identification of caudal edge of the clivus.
Identification of caudal edge of the clivus.
 Sharp disinsertion of the atlanto-occipital membrane.
Sharp disinsertion of the atlanto-occipital membrane.
 Drilling of the anterior edge of the foramen magnum.
Drilling of the anterior edge of the foramen magnum.
 Drilling of the occipital condyles: their medial one third is bounded by the hypoglossal canal. It is about 15 mm from the midline and the change from cancellous to cortical bone should be carefully appreciated during drilling. Furthermore, its opening will cause bleeding from the venous plexus surrounding the nerve.
Drilling of the occipital condyles: their medial one third is bounded by the hypoglossal canal. It is about 15 mm from the midline and the change from cancellous to cortical bone should be carefully appreciated during drilling. Furthermore, its opening will cause bleeding from the venous plexus surrounding the nerve.
 Dura opening: the tectorial membrane, attached dura, and the longitudinal part of the cruciform ligament are incised en bloc along the midline. The tectorial membrane represents the superior extension of the posterior longitudinal ligament. The basilar venous sinus should be managed when opening the dura.
Dura opening: the tectorial membrane, attached dura, and the longitudinal part of the cruciform ligament are incised en bloc along the midline. The tectorial membrane represents the superior extension of the posterior longitudinal ligament. The basilar venous sinus should be managed when opening the dura.
There are three lateral boundaries of the surgical corridor:
Transodontoid Approach
The endoscopic endonasal exposure of the craniovertebral junction and cervical spine is limited inferiorly by the projection of the nasopalatine line; it is a sagittal line tangential to the inferior edge of the nasal bones and the posterior edge of the hard palate.25,26,79,88–91 Lateral exposure is limited by the eustachian tube, which guards the parapharyngeal ICA, and by the vertebral artery (VA) and hypoglossal nerve.28
Extradural craniocervical decompression, mainly related to rheumatoid arthritis, has been performed with interesting results.28,92–95 Inherent instability of the craniovertebral junction may require posterior fusion to the occiput. Intradural extension of chordomas as well as meningiomas and even aneurysms can be treated.96,97
 Identification of the anterior arch of C1
Identification of the anterior arch of C1
 Removal of C1 with drill and Kerrison rongeur
Removal of C1 with drill and Kerrison rongeur
 Drilling of the dens after image guidance confirmation
Drilling of the dens after image guidance confirmation
 Cut of the apical and alar ligaments
Cut of the apical and alar ligaments
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree