Risk factors (predictors of low bone mass)
Inconsistent predictors of low bone mass
Female sex
Levels of exercise in childhood and adolescence
Increased age
Use of alcohol- and caffeine-containing beverages
Estrogen deficiency
Late menarche
White race
Early menopause
Low weight and body mass index (BMI)
Low endogenous estrogen levels
Family history of osteoporosis
Smoking
History of prior fracture (hip, vertebral) [1]
Table 16.2
Secondary osteoporosis
Secondary osteoporosis |
|---|
Genetic |
Hypogonadal states |
Endocrine disorders |
GI disease |
Hematologic disorder |
Connective tissue disease |
Nutritional deficiency |
Drugs |
Congestive heart failure (CHF) |
End-stage renal disease (ESRD) |
Alcoholism |
For men, 30–60 % of osteoporosis cases are associated with secondary causes [1], the most common causes being hypogonadism, glucocorticoids, and alcoholism. In perimenopausal women, the most common causes are hypoestrogenemia, glucocorticoids, thyroid hormone excess, and anticonvulsant therapy [1].
Glucocorticoids are the most common cause of drug-related osteoporosis especially long-term administration for rheumatoid arthritis (RA) and chronic obstructive pulmonary disease (COPD). In a prospective study, a group of patients was treated with 10 mg of prednisone/day for 20 weeks and experienced an 8 % loss of BMD in the spine. In addition, other secondary causes including organ transplantation, cystic fibrosis, celiac disease, and inflammatory bowel disease are conditions associated with malabsorption and resultant osteoporosis [1].
The WHO has selected BMD measurements to establish criteria for the diagnosis of osteoporosis. T-score is defined as the number of standard deviations (SD) above or below the average BMD value for a young healthy white woman. T-score is to be distinguished from a Z-score which is defined as the number of SDs above or below the average BMD for age- and sex-matched controls. According to the WHO, osteoporosis is present when the T-score is below 2.5 standard deviations. T-scores were based originally on BMD obtained by dual-energy x-ray absorptiometry (DEXA) [1] (Fig. 16.1).
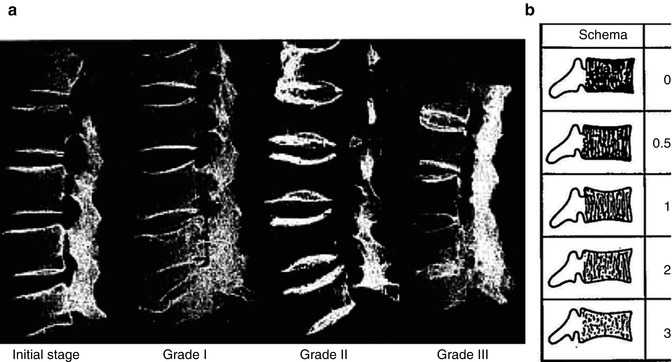

Fig. 16.1
Jikei osteoporosis grading scale. (a) Radiographic image in each grade. (b) Schema of Jikei osteoporosis grading
On the basis of simple lateral lumbar vertebral plain films, the authors proposed a grading scale to categorize the severity of osteoporosis. The classification consists of five grades: normal, initial stage, Grade 1, Grade 2, and Grade 3 (Table 16.3).
Table 16.3
Jikei osteoporosis grading scale
Jikei osteoporosis grading scale | |
|---|---|
0 | Normal trabecular pattern |
0.5 | Number of trabecula normal, bone density decreased, trabecula thin |
1 | Transverse trabecula decreased, vertical trabecula, and end plate prominent |
2 | Transverse trabecula more decrease, vertical trabecula decreased |
3 | Transverse trabecula almost disappear, vertical trabecula more like a ground glass image |
Osteoporosis plays a significant role in the progression of adult spinal instability and deformity. It has become a growing concern among the medical community as both a primary cause of musculoskeletal dysfunction and a comorbidity among patients requiring surgical intervention. An increased elderly population in industrial countries is a well-know problem to society and health services. In 2050, 54 % of the population will be older than 65 years in countries with a human development index of > 0.9 [3]. Scoliotic deformities are prevalent in 36–48 % of osteoporotic women and worsened by osteoporotic vertebral fractures [4]. Osteoporotic patients requiring spinal instrumentation for instability or deformity are of significant concern. Not long ago, patients with osteoporosis and progressive deformity (scoliosis) or fracture, even with neurological manifestations, were considered inoperable. With advances in surgical technique and instrumentation and growing expectations of patients, surgeons are taking on greater reconstructive challenges.
16.1 Instrumenting the Osteoporotic Spine
Failure of pedicle screw fixation can result from screw loosening or pullout. As posterior pedicle screw systems increase in strength and rigidity, greater demands are placed on the bone-screw interface [5]. Interface strength can be affected by surgical insertion technique, type of implant used, augmentation with bone or bone cement, and bone density [5–10] (Table 16.4).
Table 16.4
Factors affecting bone-screw interface strength
Interface strength |
|---|
Insertion technique |
Type of implant |
Bone density |
Augmentation |
In the osteoporotic spine or in revision surgery, the bone-screw interface strength may be severely compromised. Previous biomechanical studies have demonstrated that pedicle screw fixation is highly correlated to BMD [7] and that increasing in screw pullout strength is possible using a variety of methods [5, 7–9]. An expandable pedicle screw design has been shown to markedly increase the pullout strength of the bone-screw interface [11]. Statistically significant increase in pullout strength was found when an expandable screw was compared with standard pedicle screws in both high and low BMD specimens [11, 12]. Although available, (Omega-21, Biomet Spine) expandable screws have fallen out of favor because of concerns for revision surgery. Alternative methods such as augmenting conventional screws with polymethyl methacrylate (PMMA) bone cement and calcium phosphate bone cements have also been shown to increase the strength of the bone-screw interface. However, fixation in the severely osteoporotic spine represents a challenge regardless of techniques.
The key to fixation lies in the strength of the purchase obtained by the screws in the pedicle and the trabecular bone of the vertebral body [13]. Osteoporosis is implicated as the cause of hardware failure at an unknown rate. Loss of purchase and screw loosening in older patients with degenerative spondylosis has been reported to occur intraoperatively at a rate of 1.7 % and postoperatively at a rate of 3.8 % [14]. The common problems are screw bending, breakage, and lucency at the bone-screw interface. A selected survey of the American Back Society showed the rate of screw loosening, and breakage was observed in 0.81 % and 2.9 % of 617 cases and ranged from 0.6 % to 11 % and 0.6 % to 25 % in a literature review [15].
The bone-screw interface is the main determinant of the stability of the screw. Screw loosening is mainly caused by cyclic caudocephalad toggling at the bone-screw interface when an axial compressive load is transmitted through the rod to the screw [16, 17]. If a screw is inadequately anchored into the vertebral body through the pedicle, loosening of the screw could lead to loss of correction and nonunion. To predict the development of screw loosening, objective assessment of the stability of the bone-screw interface is a critical issue. If surgeons could forecast which patients are likely to develop screw loosening with the potential for loss of correction and nonunion, they may choose to use supplementary augmentation.
Bone mineral density affects the stability of pedicle screws in vivo [18, 19]. Wittenberg demonstrated that loosening occurs in cadaveric spine with BMD below 0.74 + −0.17 g/cc under physiological loading [8]. However, specified thresholds of BMD have rarely been identified below which screw loosening and nonunion develop in clinical practice. Based on in vivo studies, Wittenberg concluded that early loosening of pedicle screw may be expected at BMD measure by quantitative CT (QCT) less than 0.9 g/cm2 [8]. Okuyama suggested that patients with a mean BMD less than 0.674 g/cm2 could indicate the need for supplementation [19] (Table 16.5).
Table 16.5
Techniques for augmenting the bone-screw interface
Techniques for augmenting the bone-screw interface |
|---|
Bicortical purchase |
Undertapping |
Offset laminar hooks |
Expandable pedicle screws |
Resorbable polymers |
Rib grafts |
Milled bone |
Matchstick bone |
Bone cement (PMMA, calcium phosphate, hydroxyapatite) |
Instrumentation without tapping |
Although Pfeifer demonstrated an increase in pullout strength of 50–70 % [9] with milled and matchstick bone, this technique and several others mentioned above do not readily lend themselves to minimally invasive surgery. Previous experience with screw fixation for severe osteoporosis indicates that it is often necessary to increase the number of vertebra fused in order to avoid instrumentation failure. However, this requires longer incisions, more screws, increased operating time, and patient morbidity.
16.2 Augmentation Techniques
Cook et al. performed an evaluation of expansile pedicle screws in vivo and in vitro [11, 12] (Fig. 16.2).
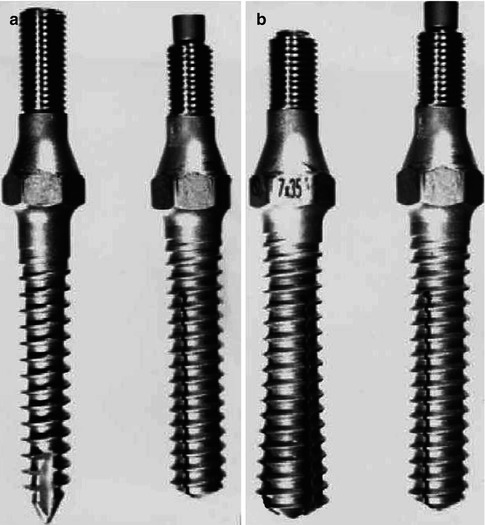

Fig. 16.2
Expansile pedicle screws
In cadaver specimens with poor BMD (0.62 + −0.44 g/cm2), the mean axial pullout strength was increased 30 % [11] with the expansile screws. The specimens were further divided into very low BMD (0.28 + −0.12 g/cm2) and high (0.95 + −0.34 g/cm2). In the very low group, the axial pullout strength was increased by approximately 50 % with the expansile design as compared to a conventional self-tapping screw [11]. In the high BMD group, the pullout strength of the expansile screw was increased approximately 200 % compared to a conventional self-tapping screw [11]. In his clinical series of 14 implanted patients, 93 % obtained relief of preoperative symptoms and 13/14 (93 %) demonstrated radiographic criteria for fusion [11]. There were no reports of screw loosening or back out. This novel technology, however, has no MIS application, and it has fallen out of favor in open surgery.
16.3 Screw Geometry/Insertion
As previously mentioned, screw effectiveness is critically dependant on its interface with bone. The principle factors that determine the magnitude of screw interface are (1) the geometry of the screw, (2) bone elastic modulus (i.e., BMD), and (3) quality of fit. Components of screw geometry that increase bone-screw interface purchase are increased major thread diameter, increased thread depth, and increased length of engagement. Screw design can be optimized for a particular site, and this approach to screw performance has been well described in the literature [20].
Screw fit can be influenced by the method of hole preparation. Based on a review of the literature, hole preparation appears to be very important in osteoporotic vertebra. Tapping pilot holes into osteoporotic bone decreases the pullout strength of screws [21, 22]. Regarding screw diameter, mean axial pullout force was increased from 459 + −183 N to 994 + −349 N just by increasing the diameter by 1 mm [8]. Zindrick evaluated the effect of the insertion depth on the number of cycles to failure. He found an increase of approximately 430 % when comparing screws inserted to 50 % of the depth of the vertebral body as compared to those inserted through the opposite cortex [23].
Screw profile and insertion are very important components to ensuring a solid bone-screw interface. Choosing a screw that will ensure good fit, has an appropriate tread pattern (cortical to engage the pedicle wall), and is inserted to the appropriate depth to reduce the likelihood of toggle-related failure are all concepts that MIS surgeons are aware of and need to be mindful of when instrumenting osteoporotic patients.
16.4 Cement Augmentation
In early evaluation of augmenting pedicle screws, Wittenberg demonstrated a 50 % increase in bending stiffness in screws augmented with PMMA and a 20 % increase in bending stiffness with their biodegradable polymer [8]. Since their report, there have been numerous studies with augmentation materials and techniques which we will review to determine the best application for MIS surgery.
There is no question that bone cement augmentation enhances the bone-screw interface strength. PMMA was used initially for pelvic surgery, and the use of bone cement in orthopedic procedures involving joint prostheses fixation has been used with consistent demonstration of an improved bone prosthesis interface [24, 25]. Today’s PMMA are radiopaque and have reduced exothermic polymerization to reduce tissue necrosis and nerve damage in case of leakage. Two cementing techniques for stabilization of a vertebra are currently in clinical use, vertebroplasty, and balloon kyphoplasty. Vertebroplasty has considerable risks regarding cement leakage and a slightly higher perioperative morbidity than balloon kyphoplasty [26].
Becker et al. [27] conducted an in vivo study on osteoporotic cadaver spines comparing augmentation techniques with PMMA. They evaluated non-augmented solid (non-cannulated) screws, perforated screw with vertebroplasty augmentation, solid screw with balloon kyphoplasty augmentation, and solid screws with vertebroplasty augmentation. They found that vertebroplasty-augmented screws, augmentation of perforated screws, and balloon kyphoplasty-augmented screws all show higher pullout resistance than non-augmented screws, but significantly higher pullout forces were only seen in the vertebroplasty-augmented group [27].
The pertinent technical comments from their study include the observation that the perforated screw had significant handling advantages. It is technically easier to inject cement directly through the screw. In addition, the screw can be positioned and verified then changed if need be, characteristics that are impossible when using a non-perforated screw. It is possible to first place screws over multiple segments then augment. They noted that a simultaneous multisegmental approach is challenging in the vertebroplasty group and nearly impossible in the balloon kyphoplasty group [27] (Fig. 16.3).


Fig. 16.3
Perforated screws
Frankel et al. also conducted a biomechanical cadaveric analysis of PMMA-augmented screws in both primary and salvage procedures [28]. They demonstrated an increase in pullout strength of 119 % in primary and 162 % in salvage procedures. This is similar to the work of Sarzier, who demonstrated an increase in pullout force of 181 % for Jikei Grade I, 206 % for Jikei Grade II, and 213 % for Jikei Grade III [10]. Importantly, Sarzier demonstrated that with augmentation, a Jikei Grade II and III vertebra exhibited pullout strength similar to levels found in non-augmented vertebrae with low-normal BMD and non-augmented Grade I vertebra, respectively [10].
Frankel also studied the effect of the volume of cement. Two groups were investigated, a low-cement group (less than 2.8 ml/pedicle) and a high-cement group (greater than 5.5 ml/pedicle). He found that cement injection less than 2.8 ml/pedicle is as effective as one that is greater than 5.5 ml/pedicle [28]. Therefore, they recommend using a lower volume of cement to reduce the likelihood of cement toxicity.
Frankel also proposed a new mechanism of introducing the cement to reduce the risk of posterior migration of cement along the injection track toward the neural elements. To overcome the availability of fenestrated screws, he designed a fenestrated tap that is commercially available (Pedestal, Abbot Spine). They first cannulated the pedicle with a Jamshidi needle then introduced a K-wire and removed the targeting needle. The bone tap was placed over the K-wire and threaded into the anterior third of the vertebral body. The tap was then flushed with 3–5 ml of saline, and cement was then injected through the tap under lateral fluoroscopy. The tap was left in place for approximately 1 min to allow partial consolidation of the cement then removed, and an appropriate screw was placed over the K-wire (Fig. 16.4).
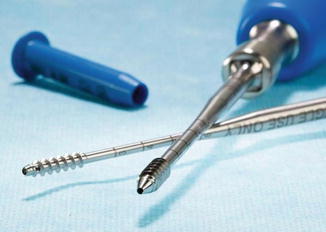

Fig. 16.4
Fenestrated tap
In a clinical series, Frankel employed his method of cement augmentation in 23 consecutive patients who all had bone softening secondary to osteoporosis and/or metastatic spinal tumor involvement [29]. Through the placement of 158 PMMA-augmented screws, asymptomatic anterior cement extravasation was observed in 39 % of patients which is consistent with what the literature reports [30–35]. They did not observe any posterior migration of cement toward the neural elements that is associated with radiculopathy that pull out strength increased by nearly 70 % when the screw was augmented with CBC [20]. Augmentation also increased stiffness by 50 % and increased the energy absorbed by cyclic loading by more than 70 % [20] Renner et al. [36] evaluated calcium phosphate cement augmentation of pedicle screws as a function and/or myelopathy. They reported one asymptomatic PMMA pulmonary embolism and one superficial wound infection. They also reported having no construct failures in their cement-augmented cases.
PMMA is not biodegradable and persists within the trabecular bone and is likely to influence bone remodeling by affecting metabolism and changing the environment. The monomer itself is toxic and can cause a large immunologic response and can cause giant cell reaction [37]. These undesirable properties have lead to the investigation of biocompatible bone cements for screw augmentation.
Lotz et al. [20] studied an injectable biocompatible carbonated apatite cancellous bone cement (CBC) that is practically non-exothermic (Norian, SRS, Skeletal Repair System, Norian Corporation Cupertino, CA). They found in vivo of injection timing and method. Using calcium phosphate cement (CaP) BoneSource (Howmedica Osteonics, Rutherford, NJ), they augmented pedicle screws and compared them to non-augmented screws and screws augmented with PMMA. BoneSource is biocompatible, osteoconductive, and resorbable and has a high 24-h wet compressive strength. PMMA was injected such that only the distal screw was augmented. CaP was injected in two different fashions. One fashion involved only the tip of the screw as in the PMMA group. The second group involved injection of CaP distally in the vertebral body as well as along the pedicle completely encasing the screw. Comparison of CaP injection by both methods to PMMA showed that PMMA produced significantly higher pullout strength in both revision and augmentation cases [36].
Yazu et al. [38] evaluated augmentation with calcium phosphate via a fenestrated screw. Their technique lends some important technical considerations to the procedure of augmentation. Using a fenestrated screw, they first injected contrast to see if there was any extravasation into the epidural venous plexus prior to injecting cement. After augmented with CPC cement, they found pullout strength to be increased by nearly 250 % [38]. They concluded that the pullout strength was similar to PMMA even though the compressive strength was not [38]. Although they demonstrated increased strength of the bone-screw interface, in vivo studies need to be conducted to determine the long-term biocompatibility, rate of resorption, as well as the long-term biomechanical behavior of the cement. In addition, calcium phosphate cement has relatively low fracture strength, is brittle, and has high susceptibility to fatigue failure [39].
Ignatius et al. [40] designed an injectable bioresorbable polymer based on alkylene bis(dilactoyl) methycrylate that has demonstrated appropriate degradation characteristics. Augmentation with the new polymer increased pullout force by 88 % in bovine vertebra and 118 % in human vertebrae [40]. In their testing, they found the mechanical efficacy comparable to PMMA, but the biodegradable properties potentially allow osteosynthesis in osteoporotic patients. However, ongoing studies to investigate in vitro and in vivo biocompatibility are needed.
Technically, the best way to cement augmenting a screw is to first place the screw and confirms the position fluoroscopically prior to augmenting. Using a fenestrated cannulated screw, this lends itself to an MIS application and is the most logical way augment screws. This also allows multiple levels to be addressed simultaneously and maximizes augmentation in regard to cement working time. McKoy and An [41] demonstrated that a cannulated fenestrated screw had a 278 % greater pullout strength than a solid screw after augmentation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








