Chapter 127 Minimally Invasive Spinal Decompression and Stabilization Techniques II
Thoracic and Lumbar Endoscopic Approaches
History
Endoscopic spine surgery refers to a rapidly evolving set of techniques potentially offering equivalent surgical outcomes with lower surgical morbidity. This includes traditional endoscopic access to a hollow cavity such as the thoracic cavity and “manufactured” cavities for spinal access. Endoscopic spine surgery does not refer to a single technique but rather to a set of tools that may be used during the approach to the spine. The philosophy behind spine endoscopy is to target the pathology and apply a therapeutic intervention while minimizing damage to surrounding nonpathologic tissues.1–3 These endoscopic techniques are part of a trend toward less (“minimally”) invasive interventions.
Endoscopic inspection of the thoracic cavity was initially conceived by Bozzini in 1806.4 Jacobaeus provided its first clinical application for diagnosing and treating tuberculosis in 1910.5 In 1991, Lewis popularized video-assisted thorascopic surgery (VATS) for pulmonary diseases. Orthopaedic applications of endoscopic principles began with the advent and acceptance of knee arthroscopy. Tagaki was the first to describe and Watanabe was the first to advance diagnostic knee arthroscopy in 1918 and 1957, respectively. Cascells in 1970 and Jackson in 1972 are credited with promoting the Japanese experience with arthroscopy in North America. Over a short time, endoscopic techniques have become standard for many abdominal and knee procedures, such as cholecystectomy and meniscectomy.6 Endoscopic spine surgery was first performed in the lumbar spine in the 1990s and, currently, experience in the lumbar spine outweighs that of thoracic endoscopic spine surgery.7 The first description of VATS for thoracic spinal diseases was published by Mack et al. in 1993.2 An endoscope (usually a 30- or 45-degree endoscope) may be placed during dorsal approaches to the thoracic and lumbar spine to improve visualization of the ventral spinal cord.
Thoracic Ventral Endoscopic Approach
Although no direct, randomized trial has compared endoscopic techniques with traditional open thoracotomy (ventral) or costotransversectomy (dorsolateral), there are many theoretical and apparent advantages to an endoscopic approach1,3,6–24 (Box 127-1). A quality endoscopic video system affords the surgeon improved visualization through outstanding illumination and up to 15× magnification. By manipulating the endoscopic portal placement, scope angle, and camera trajectory, a parallel approach to disc space can be maintained even with significant kyphotic or coronal plane deformity, making the technique useful for thoracic scoliosis correction.
BOX 127-1 Advantages and Disadvantages of Thoracic Endoscopic Spine Surgery
Advantages
Disadvantages
Surgical novelty (learning curve)
Monocular visualization (triangulation, depth perception)
Loss of tactile feedback (increased working distance)
Technical limitations in treating intraoperative complications
Anesthetic demands of double-lumen intubation and single-lung ventilation
Currently limited ability to perform endoscopic reconstruction
Currently limited ability to perform dural repair
VATS requires less muscle dissection and no rib spreading, and, therefore, it probably decreases incisional pain. Decreased soft tissue/paraspinal muscle injury also allows more cosmetically acceptable scars, lower risk of postoperative infection, and decreased compromise of respiratory and shoulder mechanics. With prone positioning, simultaneous ventral and dorsal procedures may be undertaken, obviating the need for repositioning. Simultaneous lumbar and thoracic corrections can also potentially be performed. These advantages, taken together, may reduce ICU and hospital stays, as well as operative morbidity.
On the other hand, VATS has several disadvantages over open surgery.1,3,6–24 First, these procedures require substantially different technical skill sets than traditional, open approaches. Although the word “steep” has been associated with the learning curve for this surgical novelty, the procedure more correctly represents a “flat” learning curve in that a significant amount of time must be spent with animal, cadaveric, and proctored cases before proceeding with independent VATS spine surgery. Endoscopy changes the surgeon’s binocular vision to monocular video-assisted vision. This loss of depth perception is compounded with a loss of tactile feedback associated with the long-handled instruments needed to pass through endoscopic portals. Working distance and instrument excursion increases from 4 to 30 cm. Visualization and manipulation of sensitive spinal structures also requires triangulation from widely separated starting points on the chest wall to a small thoracic disc space. Prior to embarking on endoscopic thoracic spine surgery, the surgeon must be familiar with open ventral spinal anatomy and surgical techniques. Ultimately, the surgical procedures are the same, but the methods are different enough to challenge even the most experienced spine surgeon. Other disadvantages are more technical in nature. In some centers, VATS is performed with a second, experienced thoracoscopic surgeon. In many circumstances when a spine surgeon is working with a thoracic surgeon, there is an increase in manpower during the VATS procedure. This commonly can increase operative times.
Thoracic Dorsal and Dorsolateral and Combined Open and Endoscopic Approaches
The majority of open thoracic and lumbar spine procedures continue to be performed from posterior approaches. The approaches offer relatively direct access to the bony elements and the spinal canal. However, canal exposure may result in symptomatic epidural fibrosis. The dissection and retraction of the paraspinal muscles may lead to dead space formation and extensor muscle disruption. Such disruption has been referred to as “fusion disease”25 and may be associated with early fatigability and other long-term symptoms. Endoscopic techniques may allow for less disruption of the posterior musculature and a smaller laminotomy. Dorsal and dorsolateral approaches are also limited by the surgeon’s ability to visualize the ventral dura mater. Use of an angled endoscope may also greatly improve visualization while decreasing soft tissue dissection and rib resection.
Combined approaches are being increasingly described in the literature. These approaches include simultaneous ventral and dorsal surgery for tumors and deformity.20,26 Combined approaches may also refer to combining endoscopic and open techniques in “mini-open” or endoscopically assisted spine procedures to exploit the advantages of both techniques.17,19
Anatomic Considerations for the Video-Assisted Thoracoscopic Surgery Approach
The rib cage and chest wall form a rigid open space in which endoscopic surgery may be performed. Unlike the abdomen, CO2 insufflation is not required to maintain a working space. Through most of the thoracic spine, ribs articulate at the disc space level. The rib number corresponds to the lower vertebral body at the disc space (e.g., the sixth rib comes off the T5-6 disc space). Because the rib comes directly off the disc space from demifacets arising on the vertebral body just above and below the disc, rib resection is required for adequate access to the posterolateral corner of the disc. In the lower thoracic region, T11 and T12, the rib head may be well caudal to the disc space, permitting unobstructed access.
The segmental vessels lie in the waist of the vertebral bodies, entering the epidural space via the foramina. When approaching the spine endoscopically, it is important not to inadvertently lacerate these vessels. Some spine procedures, including corpectomy and instrumentation, require unilateral and occasionally bilateral sacrifice of the segmental vessels. Discectomy and ventral release procedures, on the other hand, usually do not require vessel sacrifice. One cadaver study demonstrated adequate discectomy without sacrifice of the intercostal or segmental vessels once an adequate mobilization of the esophagus and azygos vein had been carried out. The authors concluded that the segmental vessels ought to be preserved to reduce the risk of spinal cord ischemia.10 In a two-phase goat study, thoracoscopic discectomy and fusion were undertaken both with and without sacrifice of the segmentals.27 In the first phase, the area of disc excision was slightly higher in the vessel-ligated group, but the investigators did not believe this to be significant. Operative times were the same. In the second phase, biomechanical testing of the resulting fusion was undertaken, and the vessel-spared group revealed less flexibility in lateral bending. The authors concluded that the segmental vessels in the thoracic spine can be effectively spared without injury during disc excision and fusion. They noted that while slightly more disc area was excised with ligation of the vessels, sparing the segmental vessels may provide a blood supply that aids in fusion. They recommended sparing the segmental vessels in patients with a higher risk for cord perfusion–related neurologic injury, such as revision surgery, severe kyphosis, paraparesis, and congenital anomalies.
These studies have been criticized for not adequately modeling the intraoperative conditions of spine deformity.3 Many authors report that sacrificing the segmental vessels provides better visualization through improved pleural reflection and more complete discectomy.21,22,28,29 In a report of 1197 procedures in which more than 6000 vessels were sacrificed, there were no adverse neurologic consequences.30 It may be reasonable to use both vessel-sparing and vessel-sacrificing techniques as a function of the clinical situation. For example, in congenital deformity cases in which spinal cord blood supplies may be anomalous, vessel sparing may be more important.21 Also, if the intervention requires the sacrifice of one or more segmental arteries in the mid to lower thoracic region, especially T9 to T11, a right-sided approach should be considered to avoid ligation of the artery of Adamkiewicz. The artery of Adamkiewicz can be visualized with either CT or MRI angiography, and this is recommended if a left-sided approach is being considered.
Other surgically important structures include the superior intercostal veins and the sympathetic chain. The veins empty into the azygos circulation at or about the T3-4 interspace.1 Branches from the sympathetic chain run over the rib heads, just below the parietal pleura.31 Over the 5th through 10th ribs, these coalesce as the greater splanchnic nerve that courses into the abdomen.
There are critical regional differences in thoracoscopic anatomy that dictate different exposure techniques. In the upper thoracic region, for example, the surgeon should elevate and support the ipsilateral arm to rotate the scapula away. Here, unless there are apical adhesions, the collapsed lung readily falls away from the spine, allowing excellent visualization.32 In the midthorax, there is more available space that allows more variation in placement of the camera and retractor ports. On the other hand, a fan retractor or strategically placed sponges are typically needed to keep the collapsed lung out of the operative field. Similarly, a second retracting port may be needed to move aside a bullous or stiff lung.32 Tilting the operating table forward may improve visualization with less forceful lung retraction. In the lower thoracic region, it may be necessary to retract the diaphragm, but here, as at the apices, lung retraction is not usually a problem.
Indications and Contraindications
Indications
The majority of endoscopic thoracic spine surgery is directed ventrally to avoid larger incisions and postthoracotomy pain. A traditional thoracotomy requires a large incision, division of the shoulder girdle musculature, rib resection, and forcible rib retraction. This approach can result in desiccation of the exposed lungs and vessels, measurable reduction in pulmonary and shoulder girdle function, postthoracotomy intercostal pain syndrome, and an unsightly scar.2,20 On the other hand, thoracoscopic approaches visualize the ventral spinal elements from the T1-2 to L1-2 disc spaces from the side of approach to the midline.14 VATS affords easier exposure of the extremes of the thoracic spine than open thoracotomy.33 For example, a T12 corpectomy can be performed without diaphragmatic incision, and T3 can be accessed without mobilizing the scapula as would be required with an open technique. However, the great vessels, heart, and lungs remain major considerations for either open or endoscopic surgical egress.
Currently, VATS may be used for a number of pathologies affecting the anterior and middle columns of the thoracic spine (Box 127-2). These include infections of the vertebral bodies, paraspinal gutter, discs, and epidural space, which can be biopsied, debrided, or drained. Thoracoscopic access is also highly suitable for tumor biopsy or piecemeal excision. A number of authors have also described the use of VATS in patients with degenerative conditions of the thoracic spine, including excision of herniated thoracic discs and fusion of painful degenerated segments. In trauma, corpectomy/decompression and stabilization procedures have been performed.1 The largest early experience with VATS has been for the correction of deformity.1 Here, a thoracoscope can be used to assist or perform ventral release surgery in moderate kyphotic or scoliotic deformities. VATS has included the pediatric patient population as well as those with neuromuscular scoliosis and adult degenerative scoliosis. Thoracic discectomy for ventral myelopathy has also become an established technique.
Contraindications
Contraindications to thoracoscopic spinal surgery include the inability to tolerate single-lung ventilation in patients with severe or acute respiratory insufficiency.24 However, VATS should be considered as an alternative for patients who are at high pulmonary risk for thoracotomy.24 VATS may decrease many of the detrimental physiologic sequelae of thoracotomy. For example, postthoracotomy rib splinting leads to atelectasis and decreased functional residual capacity.34 Thus, although VATS is ideally avoided in patients with severe lung disease, its less deleterious effect on pulmonary mechanics may make it a better option than open thoracotomy. Typical patient groups include those with chronic obstructive pulmonary disease; pulmonary interstitial fibrosis; or significant restrictive lung disease from thoracic spinal deformity, such as children with neuromuscular scoliosis.
Anesthesia
Cooperation between the anesthesia team and a thoracoscopic spine surgeon begins with a careful preoperative evaluation, followed by meticulous room and intubation setup. An experienced anesthesiologist with expertise in thoracic surgery is strongly recommended. Selective double-lumen endotracheal intubation allows collapse of the lung on the operative side. The tube may easily migrate, and frequent bronchoscopic assessment of tube position is mandatory.35 In small patients (<45 kg), even the smallest double-lumen endotracheal tube may not fit. In these cases, bronchial blockers are required.3,21 Blockers are technically more difficult to use and have a higher rate of incomplete lung deflation, which may seriously impair visualization.3 Prone positioning in deformity cases may allow single-lumen intubation.36
General anesthetic options include total intravenous technique, isolated volatile agent, or a combination of volatile and intravenous agents. Initially, intravenous technique was recommended because of the potential risk of hypoxic pulmonary vasoconstriction with inhalational agents during single-lung ventilation. Recently, however, a series of 85 patients found no difference among these techniques.35 Hemodynamic monitoring includes a double-lumen central venous catheter. Alternatively, pulmonary artery catheterization may be undertaken.35 Hypotensive anesthesia should be avoided in myelopathic patients or in those undergoing segmental artery sacrifice.23
Once the tube position has been confirmed, the ipsilateral lung is deflated by clamping the lumen of its endotracheal tube. Once the chest has been entered with the first portal, the lung should collapse. If inadequate collapse is noted, a short period of positive pressure CO2 insufflation may assist in collapsing the lung. Usually, this insufflation is not necessary.37
Patient and Operating Room Positioning
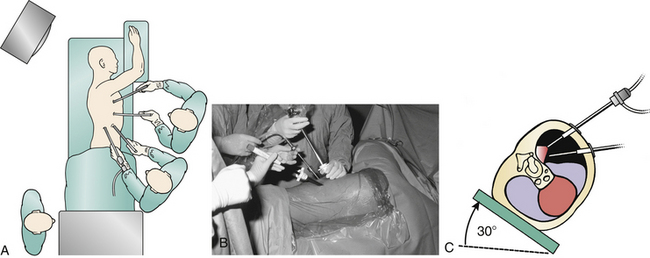
The patient is belted into place so that the table may be tilted into the Trendelenburg or reverse Trendelenburg position or even tilted right or left as necessary to improve intraoperative visualization. It is important to ensure that the patient stays in a strict lateral position during the initial approach to the spine to maintain surgeon orientation. Some surgeons prefer to “airplane” the table up for portions of the procedure, but given the loss of tactile feedback and three-dimensional information associated with endoscopy, a strict knowledge of the patient’s body position in space is necessary. In some settings, particularly in the lower thoracic spine, it may be useful to “jackknife” the table to improve access to the lateral body wall. However, in patients with significant spinal cord compression, excessive “jackknifing” may increase the risk of iatrogenic neurologic progression.37
Prone positioning may be used as part of a simultaneous ventral/dorsal approach.20,36 Simultaneous surgery eliminates the need to stage the procedures, as well as the added time and costs for repositioning, redraping, and a new operating room setup. Prone position is particularly advantageous in cases of marked instability. For example, in ankylosing spondylitis or trauma, the spine may suddenly translate at the osteotomy site during repositioning. Prone position also allows a gravity reduction of hyperkyphosis.36 Finally, prone positioning confers the additional advantage of allowing the lungs to fall away from the spine, decreasing the need for retraction.
A large operating room is typically needed to accommodate the special equipment required in VATS cases.33 The gowned and sterile team typically includes two surgeons, one assistant, and one scrub nurse. Additional personnel include the anesthesia team, somatosensory-evoked potential monitoring personnel, cell saver transfusion technicians, and circulating nursing staff. Beyond the usual complement of anesthesia machines and back tables for instruments and implants, endoscopic spine surgery requires two video monitors, a fluoroscope, and neurologic monitoring equipment (usually sensory-evoked and motor-evoked potentials). The endoscopic surgeon and spine surgeon typically work on the same side of the patient (facing the patient in the lateral decubitus position). Alternatively, they can work opposite one another. Video mixers can convert image orientation so that no mirror image instrument manipulation is required.1 More and more frequently, voice-activated robotics are being used to replace the assistant surgeon in camera positioning. Robotics may improve visualization by providing a steady image.
Instruments for an open thoracotomy should be readily available for emergency use. In the prone position, open access can be achieved with an extended costotransversectomy approach. Because the lung has already been mobilized, it is easy to enter the chest. Once in, the surgeon is readily able to access, identify, and control the major vascular structures.20 Immediate potential access to a thoracic surgeon is also recommended.
Equipment
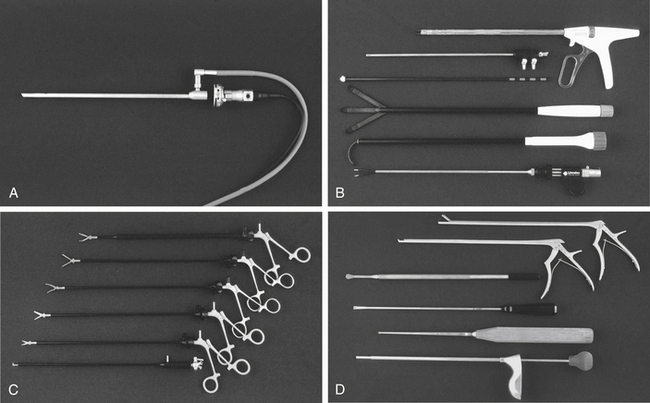
The workhorse of spinal endoscopy is the video equipment. This begins with the endoscope itself. A number of standard 10-mm endoscopes are used, most commonly 0-degree and 30-degree scopes. Occasionally, 70-degree scopes are needed. The 0-degree scope is standard for thoracoscopy, but a 30-degree scope decreases instrument crowding and allows better visualization around bony corners.24,38 Typically, a 10-mm, 15-inch end-viewing scope is used; however, in pediatric cases and, with increasing frequency in adults, a 5-mm scope may be preferred.24 The smaller scope provides less illumination, but with improvements in high-resolution, three-chip technology is adequate in most cases. Three-dimensional endoscopes are becoming increasingly available and help visualize landmarks and render improved depth perception.24
Instruments are introduced into the chest through trocars. Initially, these trocars were hard. Hard trocars may protect the thoracoscope against the rigid fulcrum of the rib cage.15 More recently, soft trocars have been developed that may be less traumatic to the neurovascular bundle on the inferior rib undersurface. Standard trocars are either 5 mm or 10 mm and are selected based on the size of instrument to be passed and the intercostal distance, which in adults is less than 12 mm.37
Typical spine instruments are customized for endoscopic applications by creating an extended shank of uniform diameter to match standard trocar sizes (Fig. 127-1). These include long-handled curets, Cobb elevators, pituitary rongeurs, disc shavers, nerve hooks, and Penfield dissectors. High-speed burs with long extenders are often required, as are more specialized types of endoscopic equipment such as Endo Shears, a bipolar endoscopic electrocautery, the harmonic scalpel, endovascular clips and loop ligatures, as well as endoscopic fan retractors.
Surgical Technique and Avoidance of Complications
A left-sided or right-sided approach to the thoracic spine may be used depending on the eccentricity of the pathology (Fig. 127-2). With a left-sided approach, the thick resilient aorta is less prone to injury than the large friable tortuous veins of the azygos system. Some authors prefer a right-sided approach when the pathology is not lateralized, because there is a greater spinal surface area lateral to the azygos vein than the aorta.14 This difference can be assessed with preoperative axial CT or MRI images. Below T9, one may consider a left-sided approach to avoid the more cranial diaphragm reflection on the right.
The initial trocar creates the main viewing portal and is placed in the anterior axillary line at the sixth or seventh intercostal space, giving an unobstructed view of the entire hemithorax. The trocar is inserted in the manner of a tube thoracostomy with blunt dissection with a Kelly forceps just over the top of the rib to avoid damage to the neurovascular bundle or deep structures. A digital exploration is undertaken to exclude adhesions and avoid parenchymal lacerations of the lung. Some recommend Bovie dissection through the musculature to prevent bleeding around portals, which can obscure the camera image.1
The camera assistant must maintain the camera orientation and keep the operative field centered in view with a steady hand. The camera and instruments should be in the same 180-degree arc to avoid working in a mirror image. The camera assistant and the operating surgeon should work in unison with small movements. The camera should be removed for cleaning at various intervals. It should be reinserted carefully, because the lung may have partially reinflated, and injury to the lung parenchyma is possible.37 Self-cleaning and defogging arthroscopes are very helpful in this regard.24
It is necessary to manipulate only one object at a time. The camera operator zooms into the operative site. Then, as new instruments are introduced, the operator pans the camera out to follow the instrument into the operative field, preferably without changing the camera angle. Similarly, retractors and other instruments should be removed under direct visualization. Fan retractors should be removed in the fully closed position only. Levering instruments on the rib cage should be avoided to decrease pressure on the intercostal nerve.39
Once in the chest, surgical levels are identified. Operating at the wrong level is always a concern in the thoracic spine. It is important to screen for variant anatomy, such as accessory ribs, with preoperative radiographs, then find the level by counting down from the first rib. The first rib may be difficult to identify. It is necessary to mark the disc space and obtain radiographic confirmation. A Steinmann pin can be passed directly through the chest wall into the pathologic level.33 A radiograph confirms level localization, and the pin also demonstrates the optimal location for the working portal. Only quality radiographic images are acceptable. Anteroposterior radiographs are typically more helpful than lateral radiographs.31 In some centers, marking beads are placed at each spinal level, and radiographs or fluoroscopy are obtained prior to entering the operating suite. The appropriate level bead is maintained on the patient for intraoperative confirmation.32
To manipulate the spinal tissues, additional instruments need to be inserted into the chest through additional trocars. Typically, two working portals are created under direct video visualization with the lung protected. The chest is percussed, and by visualizing the percussions from within the chest, additional sites are chosen. As an alternative, 18-gauge spinal needles can be placed through the interspace to verify the level and trajectory. It is necessary to organize the remaining portals to center the instruments at the level of pathology. A number of portal patterns have been described and are covered in more detail in subsequent sections. Typically, an L or V shape at the dorsal axillary line two interspaces cephalad and caudad to the viewing portal is created, depending on the chest wall morphology and the intended spinal level. Portals should be spaced far enough apart that instruments do not “fence” with each other. The final viewing portal should be far enough away from the lesion to allow a panoramic view and to allow room to manipulate instruments.3 During the procedure, the instruments and scope are interchanged between the portals to facilitate work at different levels. It is preferable to make another portal perpendicular to the operative level than to operate at an acute angle to the pathology.32
When the appropriate portals have been placed and the correct level identified, the surgeon incises the parietal pleura with monopolar cautery. Alternatively, he or she uses the harmonic scalpel to dissect with less smoke and char.12,40 It is necessary to avoid monopolar cautery over the inferior margin of the rib head, where electrocautery injury to the intercostal nerve may occur.37 The degree of pleural dissection depends on the extent of the intended surgery but may include longitudinal approaches parallel to the spine or transverse approaches parallel to each disc space. If the segmental vessels are to be preserved, smaller pleural incisions are created parallel to the disc space. Bluntly dissect the incised parietal pleura rostrally, caudally, and ventrally to expose as much of the vertebral margins as is necessary.
At the conclusion of the procedure, it is necessary to irrigate out any disc or bony debris. Most authors do not attempt to close the parietal pleura. Some recommend an intercostal bupivacaine block to decrease postoperative pain.9
A chest tube is then selected—20F, 24F, or 28F—depending on the patient’s chest size. One inserts the tube through the inferiormost portal and runs it cranially along the vertebral column. Rosenthal uses two chest tubes: one, an apical tube for air and second, a basilar tube for effusions.41 Depending on the nature of the procedure, the tube can be maintained at water seal or at 20 cm of water suction. Typically, the tube can be removed 1 to 2 days postoperatively.
In the postoperative period, most patients are extubated immediately. A chest radiograph is obtained in the recovery room to verify full inflation of the lungs.7 A brief period of postoperative ICU monitoring is recommended. Aggressive respiratory care and physical therapy are required to prevent “down lung” atelectasis and pneumonia.
Results and Complications
Anesthesia Issues
Liske et al.35 described the anesthesia outcomes in their series of 82 patients who underwent thoracoscopic spine surgery under single-lung ventilation. The authors found that the anesthesia time was not significantly different than that for their series of patients who underwent open thoracotomy. They noted that VATS required extremely long single-lung ventilation times (mean, 270.2 minutes), a significantly longer period compared with open procedures. Also, the oxygenation index decreased significantly after initiation of single-lung ventilation. Despite these physiologic stressors, the authors concluded that VATS was a reasonable alternative to open thoracotomy from the anesthesia perspective because of the clinical benefits of accelerated return to activity as well as decreased ICU and hospital stays.
Other Complications
The types of complications associated with thoracoscopically assisted spine surgery in humans are essentially the same as with an open thoracotomy approach and can be categorized as incomplete operation, neurologic injury, lung injury, and vascular injury.16,37,41 As in any spinal cord level procedure, dural laceration, cord injury, or ischemia is possible. Most common, however, is intercostal neuralgia. This may be seen in up to 21% of patients, but it is usually transient.37 Flexible portals may reduce its incidence, but intercostal neuralgia still occurred in two cases in a series of 17 patients in which these portals were used.16 Transection of the sympathetic chain causes little or no morbidity, but the surgeon should inform the patient and family of possible temperature and skin color changes below and ipsilateral to the level of surgery.
A variety of pulmonary complications have been reported. Longer periods of lung collapse increase pulmonary morbidity. To decrease the rate of prolonged atelectasis, the deflated lung should be reinflated for 5 to 10 minutes for every hour of operating time.7 Trocars or instruments may cause direct trauma to the lungs. Larger air leaks should be repaired with an endoscopic suture ligature. Other common postoperative lung problems include pleural effusions and diaphragmatic injury.
More unusual pulmonary complications may stem from anesthesia or single-lung ventilation mishaps. Sucato and Girgis42 reported a case of an 11-year-old patient with severe scoliosis who developed air in both chest cavities, mediastinum, peritoneum, retroperitoneum, and subcutaneous tissue after intubation with a double-lumen endotracheal tube. Although the patient remained hemodynamically stable, bilateral chest tubes were required. The authors note that just as for the surgeon, there is a significant learning curve for the anesthesiologist to become adept at obtaining and maintaining single-lung ventilation.
McAfee et al. reported their complications with VATS in a prospective series of 78 cases. Transient intercostal neuralgias were noted in six patients and atelectasis in five patients. One case was converted to an open procedure for extensive pleural adhesions. One case of partial spinal cord neurologic deficit was noted.37 Huang et al.39 reported their complications in a series of 90 consecutive patients treated with thoracoscopic techniques for a variety of pathologies, including infection, fracture, deformity, and degenerative disease. A total of 30 complications was noted in 22 patients (24.4%). Two of these complications were fatal, including one case of massive blood loss and another of pneumonia. One graft dislodgement required revision surgery. The other complications were transient and included four cases of intercostal neuralgia, three superficial wound infections, three cases of pharyngeal pain, two cases of atelectasis, and two cases of residual pneumothorax. Four cases were converted to open thoracotomy.
Video-Assisted Thorascopic Surgery in Deformity and Scoliosis Surgery
Indications and Contraindications
Endoscopic surgery may directly or indirectly address several of the goals of surgery for spine deformity, which include arrest of curve progression, maximization and maintenance of curve correction, improvement in fusion rate, and decompression and protection of the neural elements43–46 (Box 127-3). In scoliosis, morphologic studies demonstrate that the anterior longitudinal ligament migrates to the concavity of the curve. The anterior longitudinal ligament and costotransverse ligaments on the concave side form a structural tether that must be released to gain maximum mobility of the spine.44 Typically, ventral approaches to the spine in deformity are indicated to release these tethers to allow correction of coronal and sagittal plane deformities.
BOX 127-3 Indications and Contraindications to Thoracoscopic Spine Surgery in Deformity
Indications
The indications are the same as for thoracotomy:
• Curves greater than 60 degrees with less than 50% correction on bending
• Curves greater than 75 degrees
• Curves requiring rebalancing into the stable zone in either the coronal or sagittal plane
• Crankshaft prevention in skeletally immature patients with curves greater than 50 degrees
• Kyphotic deformities greater than 70 degrees
• Progressive congenital deformities requiring epiphysiodesis
• Patients at high risk for pseudarthrosis from dorsal fusion alone
• Patients for whom bone grafting will achieve an interbody fusion
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree