Fig. 1
OxyHb and HB-EGF suppressed K V currents of cerebral artery myocytes from control animals but not from SAH model animals. (a) Example of whole-cell K+ currents before and after 10 min application of OxyHb (10 μM) to cerebral artery myocytes isolated from control and SAH model animals. (b) Summary of 4-AP-sensitive K V currents obtained from control (n = 6) and SAH model (n = 4) animals. (c) Examples of whole-cell K+ currents before and after 10 min application of HB-EGF (30 ng/ml) to cerebral artery myocytes isolated from control and SAH model animals. (d) Summary data demonstrating that OxyHb and HB-EGF significantly suppressed K V currents in cerebral artery myocytes from control, but not SAH model animals. OxyHb treatment: control n = 7, SAH n = 4; HB-EGF treatment: control n = 6, SAH n = 5. **P < 0.01 vs control, unpaired Student’s t-test
Our previous work has demonstrated that acute application of the blood component OxyHb decreased K V currents in cerebral artery myocytes through a mechanism involving HB-EGF shedding and EGFR activation [11]. As with this previous work, OxyHb decreased K V currents in myocytes isolated from control animals. However, OxyHb failed to reduce K V currents obtained from SAH animals (Fig. 1a, d); indicating that acute application of OxyHb and 4-day exposure of subarachnoid blood in vivo may work through a common mechanism to decrease K V channel activity. Further, exogenous application of HB-EGF mimicked the actions of acute application of OxyHb, causing a reduction in K V currents in myocytes obtained from control, but not SAH model animals (Fig. 1c, d). This data suggests SAH-induced K V suppression involves HB-EGF shedding and EGFR activation in a manner similar to that caused by OxyHb.
MMP-2 Activity Is Enhanced in Cerebral Arteries from SAH Model Animals
The matrix metalloprotease subtype, MMP-2, has been implicated in HB-EGF shedding and mesenteric artery constriction [12]. In addition, our previous work has demonstrated that OxyHb increases MMP-2 activity in cerebral artery homogenates from control animals [11]. To examine if increased MMP-2 activity may be involved in SAH-induced HB-EGF shedding and K V current suppression, zymography using the MMP-2 substrate gelatin was performed using cerebral artery homogenates from control and SAH model animals. Gelatin zymography from cerebral artery homogenates produced a single 65-kDa band similar to commercially purified MMP-2 (Fig. 2a). Interestingly, MMP-2 band intensity was significantly greater in homogenates from SAH model animals (Fig. 2b, c). Although MMP-2 activity was increased after SAH, mRNA levels of MMP-2 were similar in cerebral arteries from control and SAH model animals (Fig. 2d). This data demonstrates that MMP-2 activity, but not expression, is increased in cerebral arteries after SAH in a manner similar to that observed with acute application of OxyHb.
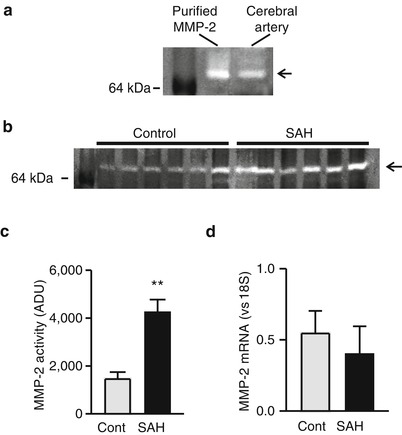

Fig. 2
MMP-2 activity is enhanced in cerebral arteries from SAH model animals. (a) Gelatin zymography demonstrating activity of commercially purified MMP-2 and cerebral artery homogenate from a control animal. (b) Gelatin zymography demonstrating enhanced MMP-2 activity in homogenates obtained from SAH model animals. (c) Summary data showing significantly greater MMP-2 activity in cerebral artery homogenates from SAH model animals compared with cerebral artery homogenates from unoperated control animals (n = 8 for each). (d) Summary data demonstrating that MMP-2 mRNA levels are similar in cerebral artery homogenates obtained from control and SAH model animals (n = 7 for each). **P < 0.01 vs control, unpaired Student’s t-test
Oxyhemoglobin Activates MMPs and Suppresses K V Currents Via ROS-Dependent and ROS-Independent Pathways
To examine whether reactive oxygen species (ROS) such as super oxide anions (O2 –) contribute to increased MMP activity caused by OxyHb, studies were performed in cerebral artery myocytes from control animals using a combination of superoxide dismutase and catalase. Superoxide dismutase catalyzes the conversion of super oxide anions (O2 –) into oxygen (O2) and hydrogen peroxide (H2O2), whereas catalase converts H2O2 to H2O and O2. The combination of superoxide dismutase (150 U/mL) and catalase (500 U/mL) decreased OxyHb-induced K V suppression by approximately 40 % (Fig. 3a, b). In comparison, the broad-spectrum MMP inhibitor, GM6001 (10 μM), caused a substantially greater decrease in OxyHb-induced K V suppression of nearly 80 %. These findings suggest that ROS partially mediates OxyHb-induced K V suppression; however, ROS-independent MMP activation also contributes to suppression of K V currents by OxyHb. Further, as illustrated in Fig. 3c, OxyHb-induced MMP-2 activity was not altered by superoxide dismutase and catalase. These findings indicate that both ROS-dependent and ROS-independent MMP activation contribute to OxyHb-induced K V current suppression and that OxyHb increases MMP-2 activity independently of ROS generation.
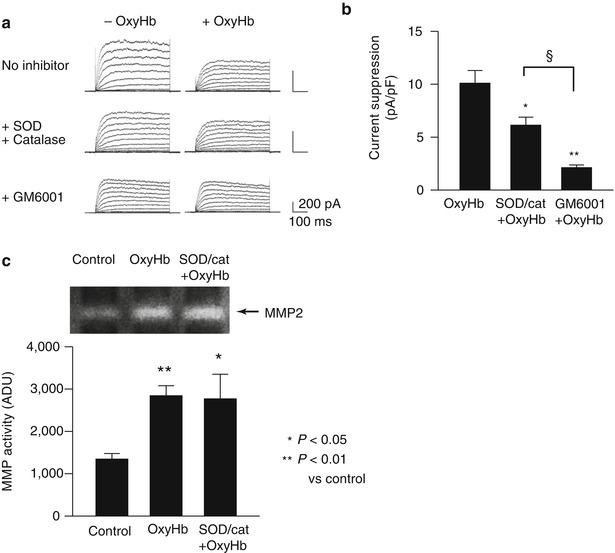

Fig. 3
ROS-dependent and ROS-independent MMP activation and K V current suppression caused by exogenous OxyHb. (a) Representative K V current recordings demonstrating the ability of the free radical scavengers superoxide dismutase (SOD) and catalase or the MMP inhibitor, GM6001 to reduce OxyHb-induced K V current suppression. (b) Summary data demonstrating that GM6001 caused a greater inhibition of OxyHb-induced K V current suppression than SOD/catalase. *P < 0.05; **P < 0.01 SOD/catalase and GM6001 on OxyHb-induced K V suppression (n = 7); §P < 0.05 GM6001 (n = 5) vs. SOD/catalase treatmtent on OxyHb-induced K V suppression (n = 5). ANOVA followed by Tukey test. (c) Representative gel and summary of zymography data demonstrating that SOD/catalase treatment did not prevent OxyHb-induced MMP-2 activation. *P < 0.05; **P < 0.01 versus control. (Control: n = 8, OxyHb: n = 8, SOD/cat + OxyHb: n = 4) ANOVA followed by Tukey test
Discussion
The present study indicates that in vivo administration of subarachnoid blood or acute ex vivo application of OxyHb act via multiple pathways that converge to induce HB-EGF shedding and K V current suppression in cerebral artery myocytes. We provide evidence that one of these pathways involves activation of MMP-2 via a mechanism independent of ROS generation and that a second pathway involves ROS-dependent activation of a matrix metalloprotease (MMP) or a disintegrin metalloprotease (ADAM) distinct from MMP-2. The combination of these ROS-dependent and ROS-independent mechanisms and the resultant shedding of HB-EGF and EGFR activation account for the selective suppression of K V channels in cerebral artery myocytes from SAH model animals.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





