Fig. 14.1
Anterior, middle, and posterior cranial fossae
Microvascular Decompression
MVD is a procedure to relieve symptoms caused by vascular compression of a nerve. When medication does not provide relief, an MVD surgery is an option to treat syndromes such as trigeminal neuralgia, hemifacial spasm, and the less common glossopharyngeal neuralgia (not discussed in this section). To gain access to the offending vessel and the affected nerve, an incision is made behind the ear on the side of the head where the patient feels pain. A portion of the skull is removed and the dura is opened to expose the cerebellum. The cerebellum is moved out of the way, exposing the brainstem. Typically under a microscope, the arachnoid layer is dissected away allowing for visualization of the facial nerve (CNVII), the vestibulocochlear nerve (CNVIII), and finally the trigeminal nerve (CNV). The surgeon places a tiny sponge between the compressing vessel and the nerve, isolating the nerve from the pulsating effect and pressure of the blood vessel (Fig. 14.2).
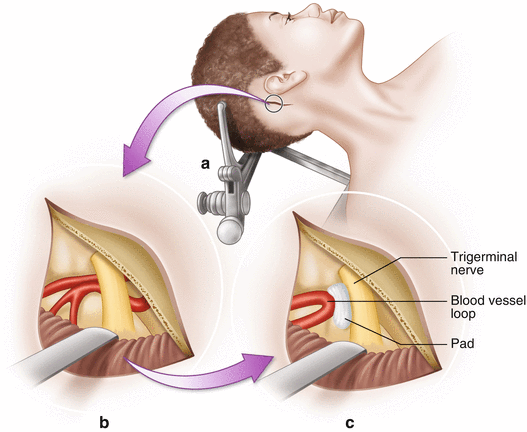

Fig. 14.2
Microvascular decompression. (a) Access to the trigeminal or facial nerve is accomplished through a posterior fossa craniotomy. (b) The cerebellum is retracted exposing the nerve and the offending blood vessel. (c) A Teflon pad is placed between the nerve and vessel, decompressing the nerve
During surgery to address cranial neuralgias, surgeons typically opt to monitor the trigeminal nerve (CNV) and the facial nerve (CNVII), using free-running EMG and triggered EMG. The trigeminal nerve is monitored by placing needle electrodes in the masseter or temporalis muscle. Facial nerve monitoring is accomplished by placing electrodes in muscles of the five main branches of CNVII that control facial expression: temporal, zygomatic, buccal, marginal mandibular, and cervical. EMG monitoring is helpful in locating cranial nerves and determining adequate decompression. A complication of MVD surgery is ipsilateral hearing loss from injury to the vestibulocochlear nerve. Brainstem auditory evoked potentials (BAEPs) are used to help prevent injury to CNVIII due to traction, ischemia, or cautery. BAEPS are also utilized when there is risk of brainstem ischemia associated with manipulation of the cerebellum.
Trigeminal neuralgia, also known as tic douloureux, is an inflammation of the trigeminal nerve causing extreme pain and muscle spasms in the face. Attacks of intense, electric shock-like facial pain can occur without warning or be triggered by touching specific areas of the face. The trigeminal nerve functions in sensing facial touch, pain, and temperature, as well as controlling muscles used for chewing. The trigeminal nerve has three major branches. The ophthalmic, or upper, branch supplies sensation to most of the scalp, forehead, eye, and eyebrow. The maxillary, or middle, branch passes through the cheek, upper jaw, top lip, teeth and gums, and to the side of the nose. The nerve’s mandibular, or lower, branch passes through the lower jaw, teeth, gums, and bottom lip. More than one nerve branch can be affected by the disorder. The superior cerebellar artery (SCA) is the vessel most often responsible for neurovascular compression of the trigeminal nerve root, although other arteries or veins may be the culprit vessels [1]. BAEPs and EMG for CNV and CNVII are typical modalities used for monitoring of MVD to relieve trigeminal neuralgia. The t-EMG response for CNV can easily be confused with CNVII responses. The latency of a t-EMG response from the trigeminal nerve should be around 5 ms, while a facial nerve response is seen around 7 ms when stimulated near the exit point from the brainstem.
Hemifacial spasm (HFS) is characterized by intermittent, involuntary twitching of the muscles in one side of the face, which lasts from a few seconds to several minutes. Spasms occur spontaneously and without warning. They are often exacerbated by stress or fatigue but can also be triggered by stimuli like sunlight, touch, chewing, and talking. Spasms do not cause pain, but can cause discomfort, impaired vision, social distraction, and embarrassment. HFS is most often caused by a branch of the posterior inferior cerebellar artery (PICA) or anterior inferior cerebellar artery (AICA), pulsating against the facial nerve root as it leaves the brainstem resulting in hyperactivity of the facial nerve [1, 2]. Similar to the treatment for trigeminal neuralgia, to relieve HFS symptoms, the facial nerve must be moved away from the offending vasculature.
To adequately monitor the facial nerve, electrodes are placed in muscles corresponding to the extracranial branches that control facial expression. For example, electrodes can be placed in the orbicularis oculi (temporal branch), nasalis (zygomatic branch), orbicularis oris (buccal branch), mentalis (mandibular branch), and if requested, the platysma (cervical branch). Free-running EMG responses in any of these muscles can indicate surgical manipulation [3]. Triggered-EMG responses can assist the surgeon in verifying the degree of decompression of the nerve. In patients with HFS, stimulation of a branch of the facial nerve may result in delayed muscle activity recorded from myotomes of adjacent branches. This is known as a lateral spread response. Current understanding is that compression of the nerve causes antidromic signals to travel back to the facial nerve nucleus within the brainstem where the nucleus becomes hyperactive and sends signals to all branches, resulting in abnormal facial movements [2–5]. Stimulation of a branch of the facial nerve may have the same effect. For example, stimulating the marginal mandibular branch and seeing a delayed response in the orbicularis oculi is evidence of a lateral spread response (Fig. 14.3). Once the offending vessel is isolated and adequate decompression has been achieved, this abnormal muscle response usually disappears. If it still persists, an additional vessel that was not apparent during visual inspection may be compressing the nerve. Monitoring the lateral spread response decreases the incidence of reoperation.
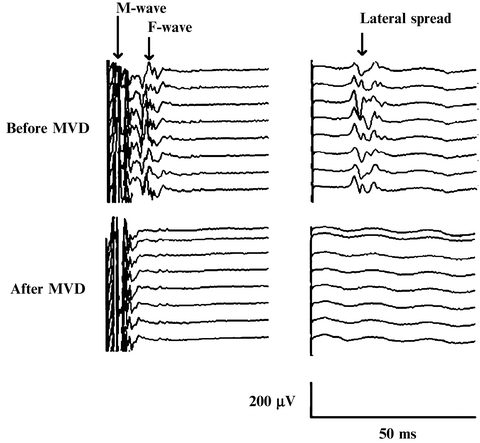

Fig. 14.3
Recordings of LSR and F wave were obtained after direct stimulation of marginal mandibular branch (left column) showing LSR to the orbicularis oculi muscle (right column). Simultaneous disappearance of the LSR and F wave after MVD was achieved. From Fernández-Conejero I, Ulkatan S, et al. Intra-operative neurophysiology during microvascular decompression for hemifacial spasm. Clin Neurophysiol. 2012;123:78–83
The close proximity of CNVIII puts hearing at risk during surgery for MVD. BAEPs during HFS surgery to protect hearing. In addition, BAEPs offer protection against ischemia to the brainstem.
Vestibular Schwannoma
A vestibular schwannoma, also referred to as an acoustic neuroma, is a benign slow growing tumor that arises from the Schwann cells covering the vestibulocochlear nerve. The vestibulocochlear nerve is the eighth cranial nerve (CNVIII) and is a sensory nerve that facilitates hearing and balance. Symptoms caused by a vestibular schwannoma correlate with the size and growth of the tumor. The most common early symptom is hearing loss. Small tumors can cause hearing loss, tinnitus, and dizziness. As the tumor expands into the cerebellopontine angle—the anatomic space between the cerebellum and the pons—hearing loss may worsen, facial weakness can occur, and balance problems may worsen. Large tumors can compress the brainstem, with severe compression causing all of the above symptoms as well as headaches and visual problems [6]. While small tumors or those causing few symptoms can be observed, surgical removal is the most common treatment for large tumors. The goal of surgery is to (1) maintain facial nerve function, (2) preserve socially useful hearing in the affected ear, and (3) remove as much tumor as possible. Total tumor removal carries a higher risk of hearing loss and facial nerve damage so surgeons often opt for partial or near-total tumor removal in order to preserve neurological function [7].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







