Motion Preservation—Disc Replacement for Lumbar Degenerative Disorders with the ProDisc®-L Prosthesis
R. Bertagnoli
The development of innovative treatment options for the treatment of degenerative disc disease (DDD) is presently more important than ever. Worldwide, the number of patients who suffer from chronic low back pain and musculoskeletal diseases is increasing. Many of the patients who are affected by back ailments can be treated with conservative therapy. But more often this treatment option fails and a surgical intervention is necessary.
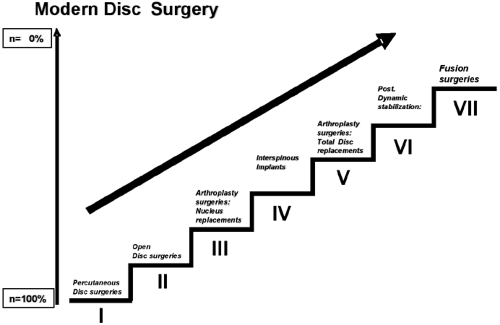
Presently there are different surgical options available, but not every intervention shows satisfactory results. Because the existing treatment options cannot reach a “restitutio ad integrum,” we should apply not the maximum possible but the most adequate treatment for the specific condition of the patient. Based on a “treatment of little and increasingly invasive therapy steps,” the therapy should ideally consist of a series of small therapy steps. The goal is to achieve a better success rate and to minimize the number of patients who undergo a more extensive surgery, with higher risks and collateral damage, by using a “negative patient selection” process (i.e., using exclusion criteria to direct patients toward an alternative form of therapy, only focusing on “nonresponders” in each category) (1,2). Along this “step algorithm” the risks and the collateral trauma caused by a surgical intervention can be kept to a minimum, and if one treatment option fails, the patient can be offered a further therapy step. Within this step algorithm, motion-preserving spine surgeries can fill the gap between decompression techniques and fusion techniques.
Presently, DDD can be treated with a modern step algorithm in seven surgical steps (Fig. 20.1): (a) percutaneous decompression techniques, (b) open decompression techniques, (c) nucleus replacement (e.g., PDN, Neudisc, DASCOR), (d) interspinous implants (e.g., Wallis, Coflex, X-Stop, Diam), (e) total disc replacement (e.g., Charité, ProDisc, Maverick, Flexicore, Mobidisc, Centurion, activL), (f) posterior dynamic stabilization (e.g., Dynesys), and (g) fusion surgeries.
Higher therapy steps mean a larger treatment size combined with higher risk factors and larger collateral damage to the surrounding structures (1,2).
Total disc replacement became more and more important over the past years as an alternative solution for patients who were not candidates for a fusion surgery or nucleotomy.
Nucleus replacement technologies are not indicated for multilevel DDD, and at this time of the investigation there is no standard definition of when the implantation will be successful (i.e., the degree of annulus degeneration and disc height loss) (3). The disadvantages of fusion surgeries include not achieving restoration of the natural disc
function and additionally eliminating the motion of the affected segment. This results in rapid degeneration of the adjacent segments by developing a transitional zone syndrome (“fusion diseases”) including discopathy, facet hypertrophy, and spinal stenosis (1).
function and additionally eliminating the motion of the affected segment. This results in rapid degeneration of the adjacent segments by developing a transitional zone syndrome (“fusion diseases”) including discopathy, facet hypertrophy, and spinal stenosis (1).
Total disc replacement is an innovative technique to restore stability without definitive destruction of the function of the motion segments, leading to pain relief with maintenance or restoration of motion.
To find the most effective treatment for each patient, careful patient selection is still the most important step before every surgical intervention.
The ProDisc-L
Inspired by the promising results of total hip and knee replacements (4), several authors designed artificial lumbar disc prostheses consisting of similar materials. In the early 1990s the first ProDisc was implanted in humans, and by 1993 it was implanted in 64 patients. The analysis of the long-term results of these patients and their success indicated that the prosthesis would have a good outcome. In 1999 the first ProDisc II (4) was released. The ProDisc-L device has been completely redesigned into a second generation. A new application technique has been developed as well, leading to a higher precision of application and ability to perform the procedure in a minimally invasive way.
The ProDisc II, a semiconstrained prosthesis, has a three-component design: two metal endplates made of cobalt-chromium-molybdenum alloy with a single central keel and a central convex weight-bearing surface made from ultrahigh molecular weight polyethylene (UHMWPE) (5,6).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree